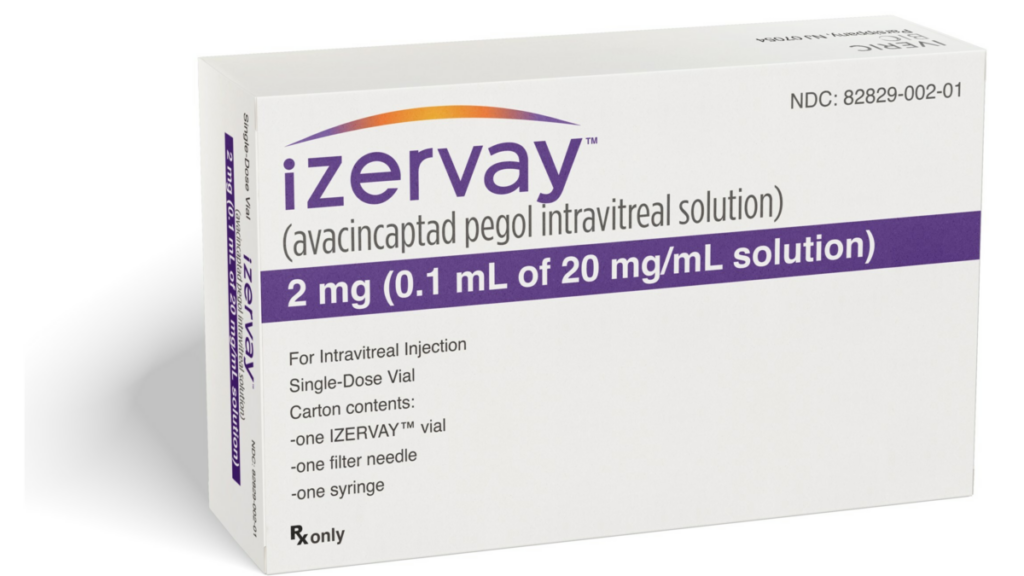
Iveric Bio, a company of Astellas, has secured recent approval from the US Food and Drug Administration (FDA) for its innovative drug, Izervay (avacincaptad pegol intravitreal solution). This major achievement now allows Izervay’s use for treating geographic atrophy, introducing a new therapeutic option for those suffering from this condition.
Izervay is the only approved treatment for geographic atrophy that has demonstrated a significant decrease in the rate of geographic atrophy progression at the 12-month primary endpoint, based on results from two rigorous Phase III clinical trials.
“Geographic atrophy has a devastating impact on patients’ lives and can lead to irreversible vision loss. As a C5 inhibitor, Izervay has shown to slow geographic atrophy progression by targeting the source of retinal cell death and may preserve the upstream benefits of the complement system. The FDA approval of Izervay is great news for the retina community and our patients suffering from geographic atrophy,” said Arshad M. Khanani, director of clinical research at Sierra Eye Associates, Reno, Nevada, in Iveric Bio’s news release.
What Is Geographic Atrophy?
Geographic atrophy is an advanced form of age-related macular degeneration (AMD), marked by the appearance of atrophic lesions starting in the outer retina and gradually expanding to cover the macula and the fovea — the central focus point of the macula. This continuous expansion results in irreversible vision degradation over time. Importantly, geographic atrophy affects an estimated 1.5 million individuals in the US.
While early stages of geographic atrophy might not cause noticeable symptoms, especially if limited to one eye, the disease’s progression results in clear symptoms, including decreased visual clarity, challenges with activities requiring central vision, a central blind spot, difficulties in low-light situations and diminished color vibrancy. This complex progression greatly impacts an individual’s visual function and overall quality of life.
XTALKS WEBINAR: AI in Clinical Trials: Leveraging a Clinical Trial Intelligence Platform
Live and On-Demand: Thursday, September 28, 2023, at 10am PDT (1pm EDT)
Register for this free webinar to learn ways to leverage artificial intelligence (AI) and machine learning (ML) technologies to optimize and predict a study’s performance. The featured speakers will share how AI can help improve enrollment forecasting, reduce protocol deviations, improve site selection and performance.
How Does Izervay Work?
Izervay contains avacincaptad pegol sodium, an effective complement C5 inhibitor. Avacincaptad pegol combines an RNA aptamer with a roughly 43-kiloDalton (kDa) branched polyethylene glycol (PEG) molecule. Avacincaptad pegol can bind to and inhibit the complement protein C5, preventing the cleavage of C5 into C5a and C5b and significantly reducing membrane attack complex (MAC) formation.
The recommended dosage of Izervay is 2 mg (equivalent to 0.1 mL of a 20 mg/mL solution), administered via intravitreal injection into each affected eye monthly for up to 12 months.
Efficacy and Safety of Izervay
The FDA’s approval of Izervay originated from a thorough evaluation of the GATHER1 and GATHER2 Phase III clinical trials. These trials investigated the safety and effectiveness of a monthly 2 mg intravitreal administration of Izervay in individuals suffering from geographic atrophy as a result of AMD.
The growth rate of geographic atrophy was evaluated at the beginning of the study, at the six-month interval and at the completion of the 12-month period. In both pivotal trials, the primary analysis revealed a statistically significant reduction in the rate of geographic atrophy progression among patients treated with Izervay, compared to those who received a sham treatment. Impressively, a slowing of the disease progression was evident as early as six months, with a substantial reduction of up to 35 percent observed within the initial year of treatment.
Throughout the clinical trials, Izervay consistently demonstrated a favorable safety profile and received positive patient tolerability feedback. In the comprehensive GATHER clinical trial series, common adverse reactions (≥ 5 percent) observed at the 12-month mark in individuals treated with a 2 mg dose of Izervay included conjunctival hemorrhage (characterized by bleeding beneath the clear eye lining: 13 percent), elevated intraocular pressure (indicating increased fluid pressure within the eye: 9 percent) and instances of blurred vision (8 percent).
What Is the Price of Izervay?
Izervay is anticipated to be available in the US market very soon, and its cost is projected to be $2,100 per vial (that is $2,100 per injection). It is worth mentioning that coverage for Izervay might be accessible through major insurance plans, contingent upon the particulars of each individual insurance policy.
Another New Treatment for Geographic Atrophy
There is one other FDA-approved drug for geographic atrophy on the market. Notably, Apellis Pharmaceuticals, a leading global biopharma company with expertise in complement therapies, has made progress in this area. The company received FDA approval for Syfovre (pegcetacoplan injection) in 2023, a treatment designed to combat geographic atrophy secondary to AMD.
Clinical data showed that Syfovre effectively reduced the rate of geographic atrophy lesion expansion compared to a placebo, demonstrating increasing efficacy over time. The most significant benefit was observed during the 18 to 24-month period, with the trial results showing a remarkable reduction of up to 36 percent in lesion growth with monthly administration, as confirmed by the DERBY trial.
Syfovre’s approval was granted due to its demonstrated capacity to slow the growth of geographic atrophy lesions (which are indicators of the disease), with no impact on the overall progression of the disease or the worsening of central vision loss.










Join or login to leave a comment
JOIN LOGIN