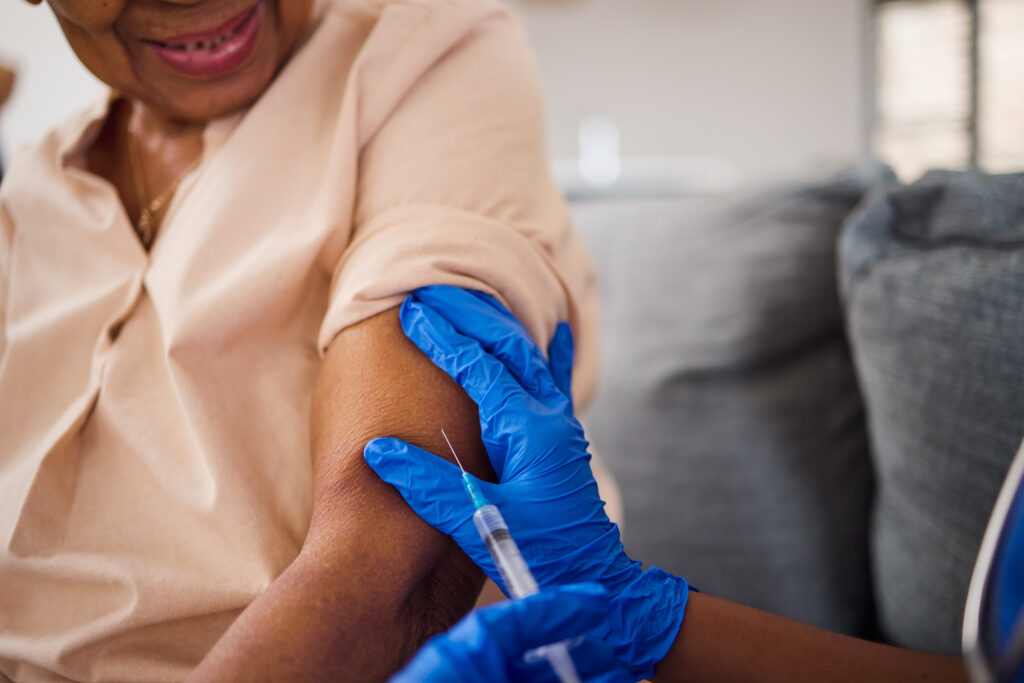
The US Food and Drug Administration (FDA) has green-lighted the first pneumonia vaccine specifically designed for adults 50 years of age and older.
The approval was granted to Merck’s 21-valent pneumococcal conjugate vaccine Capvaxive (formerly V116) for the prevention of invasive pneumococcal disease (IPD) and pneumococcal pneumonia in adults.
While Merck says the vaccine is specifically designed for adults and covers serotypes that cause about 84 percent of IPD in adults 50 years of age and older, it is officially approved for adults 18 years of age and older.
This is more than the approximately 52 percent covered by Pfizer’s Prevnar 20, which is the current pneumococcal vaccine market leader and the top player in the company’s pneumococcal vaccine portfolio.
These numbers do not reflect the efficacies of the vaccines, which have not been compared head-to-head in any trial yet.
In the US, more than 150,000 adults are hospitalized with pneumococcal disease each year. It can cause serious complications such as bacteremia and pneumococcal meningitis and the case death rate for those hospitalized is 14 percent.
XTALKS WEBINAR: Streamline Serological Testing Using Multiplexed Assays that Generate Multiple Results in a Single Test
Live and On-Demand: Tuesday, July 16, 2024, at 11am EDT (4pm BST/UK)
Register for this free webinar to gain insights into the transformative capabilities of an innovative, multiplexed microarray-based technology for serological testing and vaccine analytics.
Pneumonia prevention has largely focused on children thus far, but in recent years, there has been a growing emphasis on preventing the disease in adults as well. However, adapting pediatric pneumococcal vaccines for adults hasn’t been straightforward, as efficacies, even with added serotypes, have not translated to the adult setting.
With better surveillance data, experts say they’ve learned that the serotypes that cause disease in children are different from the ones that cause it in adults. This has allowed for the design of vaccines specifically for adult populations.
Merck is looking to target a range of age demographics, including adults 65 years of age and older.
Capvaxive received accelerated approval, which means Merck will have to verify its clinical benefit in a confirmatory trial. The US Centers for Disease Control and Prevention (CDC) will have its Advisory Committee on Immunization Practices (ACIP) convene on June 27 to discuss recommendations for the use of Capvaxive in adults.
Capvaxive’s approval was partially based on data from four Phase III trials including the STRIDE-3 study, which found that the vaccine was non-inferior to comparator vaccine Prevnar 20.
According to a November 2023 readout, Capvaxive also elicited superior immune responses for ten of the 11 serotypes not covered by Prevnar 20.
Additional data from the Phase III STRIDE-4 and STRIDE-6 studies, which assessed Capvaxive in vaccine-naïve and vaccine-experienced adults, also helped seal the approval.
Other companies are also gearing up for their own adult-targeted pneumococcal vaccines. This includes California’s Vaxcyte, which is developing 24-valent and 31-valent shots. The 24-valent vaccine covers all of the serotypes in Prevnar 20 along with four unique strains. The vaccine has done well in a Phase II trial against the Pfizer vaccine.
Pfizer’s Prevnar 20 was first approved in 2021 for adults 18 years of age and older and has since dominated the market. In April 2023, it received a label expansion from the FDA for use in infants and children six weeks through 17 years of age.
Despite Pfizer’s market dominance with its billion-dollar Prevnar franchise that includes Prevnar 13 which Prevnar 20 was built off of, Merck’s two pneumococcal vaccines, Pneumovax 23 and 15-valent Vaxneuvance (formerly V114), haven’t been doing too shabby. In 2023, Vaxneuvance, which was approved in 2021, netted $665 million in sales.
Last year, sales of Pfizer’s pneumococcal vaccines reached $6.4 billion. However, Vaxneuvance has contributed to slowing down Prevnar 20’s sales growth from 20 percent in 2022 to two percent last year.
If you want your company to be featured on Xtalks.com, please email [email protected].






Join or login to leave a comment
JOIN LOGIN