Obesity remains one of the most significant public health challenges of the 21st century, not only due to its alarming prevalence but also because of its vast implications on individual and societal health.
Recent statistics from the World Health Organization (WHO) reveal that worldwide obesity has nearly tripled since 1975. The latest reports indicate that over 650 million adults and an increasing number of children and adolescents are classified as obese, highlighting a global epidemic that spans continents and cultures.
The US Centers for Disease Control and Prevention (CDC) reports that approximately 42.4 percent of adults in the US are living with obesity, with notable variations observed across age, ethnicity and socio-economic groups. In 2019, the estimated cost of medical care related to obesity in the US was nearly $173 billion annually.
Obesity is associated with numerous health risks, including higher instances of chronic conditions such as diabetes, heart disease and various forms of cancer, making it a leading cause of preventable deaths worldwide.
Given these dire statistics, the need for innovative approaches in obesity clinical trials has never been more urgent. These trials are crucial for understanding and managing obesity while improving strategies to combat this complex health issue.
Dr. Amanda Sylvester, DNP, APRN, CPNP-BC, Advanced Clinical Practitioner at Medpace, emphasizes the need for a balanced approach in these trials, stating, “It’s important to balance the patient-centric approach with protecting the study’s endpoints.” This strategy ensures that trials prioritize both patient experiences and scientific rigor, aiming to reshape the future of obesity management.
In an informative webinar led by Dr. Sylvester and her colleagues at Medpace—Jennifer Brown, Sr. Director of Clinical Trial Management, and Prudence King, MSN, APRN, AGPCNP-BC, Advanced Clinical Practitioner— they explore the importance of a patient-centric approach in the design of clinical trials for patients with obesity.
Read on to discover key lessons on patient engagement and retention in obesity clinical trials, showcasing Medpace’s innovative, patient-focused strategies to improve healthcare outcomes.
Challenges in Obesity Clinical Trials
Conducting obesity clinical trials presents unique challenges that reflect the complexity of the disease itself. Trials must manage diverse participant pools while addressing psychological expectations, comorbid conditions and the need for high data integrity and patient-centered care.
Recruitment and Selection of Participants
Obesity affects people across different ages, ethnicities and socioeconomic backgrounds, each with distinct genetic, environmental and behavioral factors contributing to their condition. This diversity requires flexible and inclusive recruitment strategies that can engage a broad participant base.
“Obesity is a complex and global public health concern, often associated with reduced life expectancy.”
— Amanda Sylvester
Identifying candidates for obesity trials requires extensive screening for comorbid conditions like diabetes, hypertension or cardiovascular disease, which can complicate eligibility or demand specialized management.
Patient Retention
Once participants are enrolled, retaining them becomes the next hurdle. Obesity trials often deal with the psychological impacts of weight loss, where participants may have high expectations for rapid results.
As Dr. Sylvester notes, “Data indicates that people with obesity make multiple attempts at losing weight throughout their journey.” This history can lead to disappointment if progress does not meet their expectations, resulting in high dropout rates.
Furthermore, the use of placebos can exacerbate retention challenges. Patients who perceive they are not receiving active treatment may lose interest in continuing, especially if they are not experiencing noticeable benefits.
Educating participants about the realistic pace of weight loss and the potential for placebo assignment is crucial to managing expectations and improving retention. Providing regular follow-up, personalized counseling and building strong rapport between participants and staff helps maintain engagement throughout the trial.
Managing Comorbidities and Side Effects
Participants with obesity often have complications like cardiovascular disease, depression, anxiety and diabetes. Managing these conditions, especially with medications that may cause weight gain, adds complexity to trials and may require tailored interventions, complicating standardization. Additionally, pharmacological interventions can introduce side effects that must be carefully managed to ensure patient health, compliance and data integrity.
Data Integrity and Clinical Outcomes
Genetic, lifestyle and metabolic differences mean that obesity treatments may not work uniformly across participants. This variability complicates the analysis of trial results, requiring sophisticated statistical methods to identify true effects.
Additionally, adherence to prescribed diet, exercise and lifestyle changes is essential for evaluating the efficacy of interventions, but monitoring these behaviors presents challenges. Effective trial designs must account for these complexities to produce meaningful outcomes.
Collaboration for Success in Obesity Trials: The Role of Advanced Clinical Practitioners (ACPs)
Addressing the complexities of obesity clinical trials requires a multifaceted approach. Multidisciplinary teams, including endocrinologists, psychiatrists, dieticians and exercise physiologists, play a crucial role in providing comprehensive care and management to participants.
At Medpace, Advanced Clinical Practitioners (ACPs) are essential in bridging clinical research with patient-centered care, enhancing the effectiveness, ethical standards and overall participant experience in obesity trials (Figure 1).

Figure 1. How Advanced Clinical Practitioners (ACPs) drive efficiency and success in obesity clinical trials.
Below is a closer look at the benefits of multifunctional collaboration in obesity trials at Medpace, with a focus on the key role ACPs play.
Clinical Expertise and Protocol Development
ACPs like Dr. Sylvester and Prudence King contribute valuable insights during the trial design phase, ensuring that protocols are scientifically sound and practically applicable. Their understanding of obesity’s physiological and psychological aspects enables them to advise on the most effective intervention strategies and assessment tools.
ACPs thoroughly review study startup documents with the patient perspective in mind, ensuring protocols are adapted to participants with obesity, who may have comorbidities and physical limitations. This approach helps make trials more inclusive and accessible.
ACPs also collaborate with regulatory affairs teams to ensure that informed consent documents are not only aligned with the protocol but are also easily understood by the patient population. By ensuring clinical accuracy and relevancy in patient-facing materials, ACPs help enhance recruitment and retention efforts.
Additionally, ACPs work closely with Medpace’s medical monitors, reviewing laboratory and safety data in aggregate to identify potential trends and ensure patient safety remains a top priority throughout the trial. This collaboration with data management teams helps prioritize query responses and further supports the integrity and safety of the study.
Supporting Site Engagement and Participant Care
“We are dedicated to supporting patients through their medical journey from the time of diagnosis throughout the course of their treatment.”
— Prudence King
ACPs play a crucial role in supporting study staff to ensure trials run smoothly and patient care is well-coordinated. ACPs collaborate with Clinical Research Associates (CRAs) and other study team members to offer comprehensive guidance on study protocols. This includes in-depth protocol reviews and helping sites navigate protocol-related challenges.
ACPs also assist in the review of patient-facing materials, ensuring they are clinically accurate, relevant and accessible to the specific study population.
By evaluating study risks and developing mitigation strategies, ACPs ensure that the clinical sites are well-prepared to manage participants. They provide study teams with the necessary tools to anticipate and address participant challenges, such as managing adverse events like injection site reactions and gastrointestinal issues.
Moreover, ACPs help sites implement retention strategies, ensuring that participants stay engaged throughout the trial, even when faced with frustrations such as weight loss plateaus or potential placebo assignments.
Enhancing Patient Recruitment and Retention
ACPs help enhance patient retention, a known challenge in obesity trials. Since patients may become disengaged if they do not see immediate weight loss, ACPs support study teams by helping ensure that patient expectations are managed from the beginning. This includes advising study teams on how to communicate potential benefits like dose escalation or the importance of completing the trial even if the participant may be on a placebo.
ACPs also aid in the development of retention strategies, such as offering open-label extensions or milestone-based incentives. They collaborate with study teams to provide sponsor-approved remedies and protocol-guided interventions to manage adverse events that may cause participants to consider dropping out.
By ensuring that these strategies are in place, ACPs help maintain participant engagement, a key factor for successful trial completion.
Training and Support for Study Teams
Training is a critical component of ACPs’ responsibilities in ensuring the success of obesity trials. ACPs provide study-specific therapeutic training tailored to the unique needs of the trial. These trainings are comprehensive and cover every aspect of the study protocol, from patient care to protocol adherence.
A notable feature of the training process is the one-on-one meetings between ACPs and CRAs prior to Site Initiation Visits (SIV) and Routine Monitoring Visits (RMV). These meetings are designed to ensure CRAs have a thorough understanding of the protocol, particularly the clinical assessments and monitoring procedures, helping to guarantee protocol fidelity at the sites (Figure 2).
“Our ACPs play a large role in training not only our Medpace team but also the site staff on key aspects of the trial to ensure data around all points is collected and processed correctly,” says Brown.
ACPs also contribute to the development of patient-centric training materials, ensuring they are clear, accessible and aligned with the needs of the study population. This may include instructional videos, written materials or a combination of both, depending on the demographics of the participants.
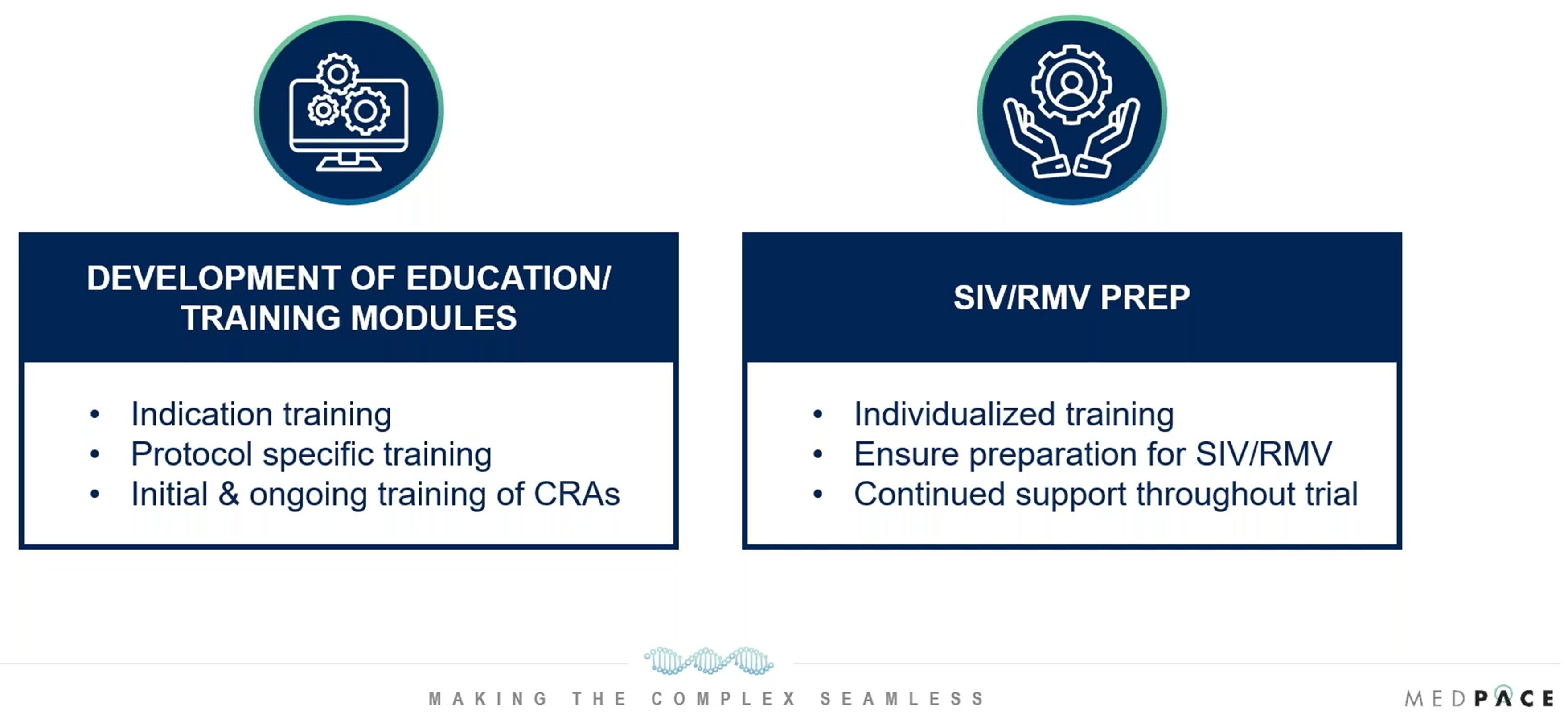
Figure 2. How Advanced Clinical Practitioners (ACPs) aid with study team training.
Throughout the trial, ACPs provide ongoing support to study teams, addressing any questions or concerns that arise. Their readiness to assist with troubleshooting unforeseen issues in real time is vital for maintaining the integrity and progress of the trial.
Advocacy and Oversight
ACPs advocate for the patient’s experience by actively participating in the design and review of patient-facing materials and processes. Their role includes reviewing informed consent documents, patient education materials and overall trial protocols with the patient’s needs and understanding in mind. This helps minimize confusion and frustration for participants, reducing the risk of dropout. ACPs’ patient-centric approach bridges the gap between clinical research and patient care, enhancing both the effectiveness of trials and the participant experience in obesity studies.
Balancing Patient-Centric Approaches with Endpoint Protection
In obesity trials, it is crucial to balance patient care with the protection of trial endpoints. A patient-centric approach ensures participants’ comfort and well-being, while endpoint protection guarantees the collection of precise, reliable data. This dual focus is crucial for the success of the trial, as it enhances both the patient experience and scientific validity.
Patient-Centric Approaches
Focusing on the well-being of participants is especially important in obesity trials, where patients may have encountered multiple weight loss challenges or have co-existing medical conditions.
As Dr. Amanda Sylvester notes, “Most people we interact with who have obesity are not attempting to lose weight for the first time,” emphasizing the emotional and psychological difficulties that often accompany these patients’ journeys. Recognizing this, ACPs play a pivotal role in reviewing patient-facing materials and developing strategies that help foster a supportive environment where patients feel understood.
ACPs also collaborate with recruitment and retention teams to ensure patient expectations are clearly communicated and materials are developed with accessibility in mind.
For example, ACPs work with Medpace’s internal design teams to create patient-friendly educational materials that effectively guide participants through each step of the trial. These resources are designed to keep patients informed and reduce confusion or frustration, thereby minimizing the likelihood of dropouts and improving adherence to the trial protocol.
Endpoint Protection
While patient experience is a priority, protecting the scientific integrity of the trial is equally critical. The success of the trial depends on the collection of accurate, reliable data to evaluate the safety and efficacy of new treatments. ACPs, alongside medical monitors, clinical trial managers, data management teams and more, work collaboratively to ensure that data collection and measurement remain consistent across all sites involved in the trial (Figure 3).
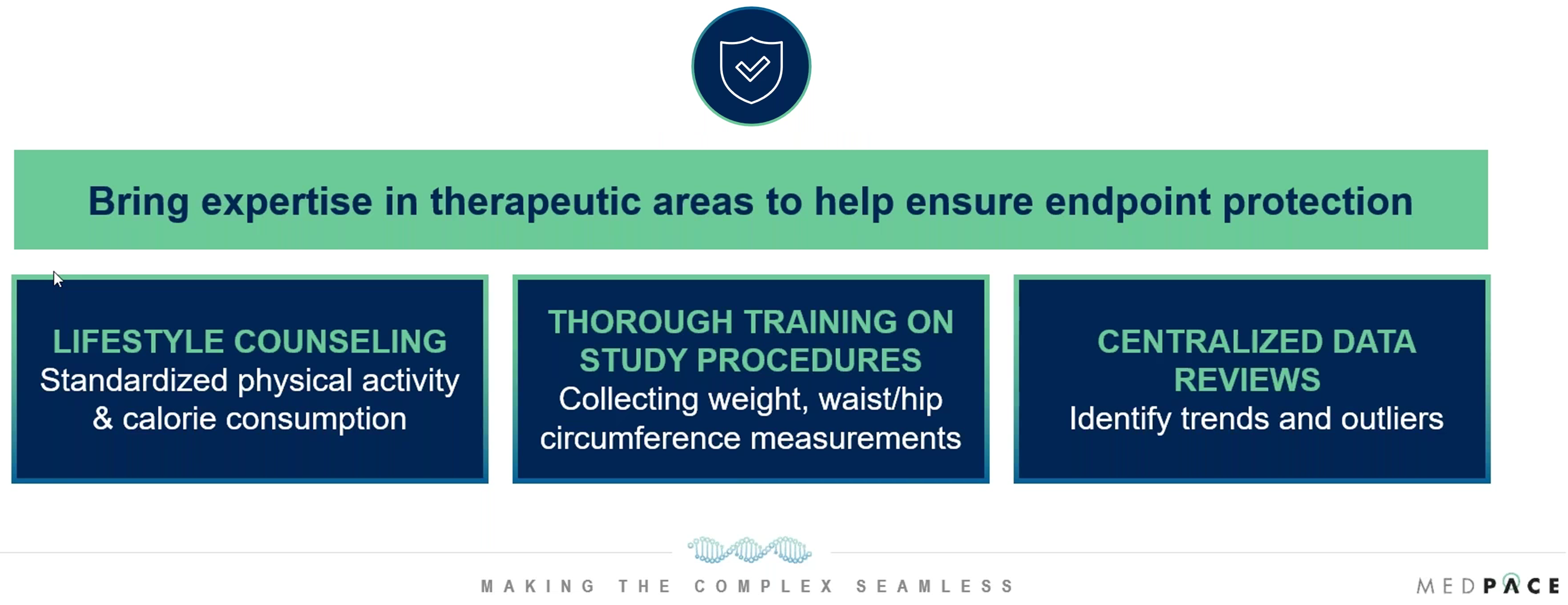
Figure 3. Methods for endpoint protection in obesity clinical trials.
This careful coordination ensures that trial data is valid, reliable and scientifically meaningful.
Combining Both Approaches
Balancing patient-centric care with endpoint protection requires designing trial protocols that meet both participant needs and scientific objectives. While patient engagement through communication is essential, these interactions must be carefully managed to avoid disrupting data collection or endpoint protection.
“Our ACPs collaborate and work closely with our teams, taking a holistic approach to data reviews and looking at it centrally to identify any trends and data outliers where our teams may need to shift their focus to ensure that we’re not compromising data quality, study-specific endpoints or subject safety.”
— Jennifer Brown
ACPs play an active role in designing frameworks that accommodate necessary patient care adjustments, such as managing side effects, without compromising data integrity or the quality of trial results. This balance ensures that both patient care and scientific rigor are maintained, leading to successful trial outcomes.
As obesity management evolves, integrating ACPs, multifunctional collaboration and patient-centered strategies is increasingly important for addressing scientific questions and the patient experience in clinical trials.
Medpace’s focus on these elements supports the development of more effective solutions for managing obesity. To learn more, be sure to watch the on-demand webinar featuring the experts from Medpace.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN