Heart failure, affecting over 64 million people worldwide, remains a formidable challenge in the medical community despite advancements in pharmacotherapy over the past decade.
The prognosis for heart failure patients is disheartening, with a mere half surviving beyond five years post-diagnosis. This stark reality highlights the urgent need for novel heart failure management options, particularly for advanced stages of the disease. The economic impact is also staggering, with heart failure leading as the top cardiac diagnosis among Medicare patients, reinforcing the demand for innovative care approaches.
“Now we recognize that over the last ten years or so there have been major developments in pharmacotherapy for the management of different etiologies of heart failure,” says Dr. Nicholas Alp, MD, PhD, FACC, FRCP, Vice President of the Medical Department at Medpace. “But we also recognize that, in the last few years in particular, there has been enormous innovation in catheter-based device therapies addressing many aspects of the mechanical considerations around heart failure.”
Dr. Alp recently moderated a webinar in which his colleagues at Medpace discussed emerging cardiovascular devices and their potential to redefine the trajectory of heart failure.
In the webinar, Dr. Adam M. Lubert, MD, Medical Director of the Medical Department at Medpace, discussed new interventions for heart failure, showcasing devices for reverse remodeling and shunt devices.
Dr. Lubert was joined by his colleague Dr. Aung Myat, MRCP, MD, FACC, FESC, Medical Director of the Medical Department at Medpace, who shared insights into the latest neuromodulation devices for heart failure.
The webinar concluded with Dr. Dean J. Kereiakes, MD, FACC, MSCAI, Vice President of the Medical Department at Medpace. Dr. Kereiakes discussed the prevalence of valvular heart disease and shared insights into catheter-based interventions targeting aortic, mitral and tricuspid valves.
Read on to learn more about the groundbreaking devices and trials shaping the future of heart failure management, as detailed by Medpace’s experts in the field.
New Interventions for Heart Failure
In recent years, the medical field has seen groundbreaking advancements in the treatment of heart failure, with a substantial focus on devices for reverse remodeling and shunt devices. These innovative approaches address the mechanical aspects of heart failure, offering new hope and improved quality of life for patients battling this challenging condition.
Physical Reverse Remodeling Devices
Physical reverse remodeling devices are innovative solutions designed to modify the structure of the heart’s ventricles in patients with heart failure. These devices operate on two principal mechanisms: partitioning the ventricle to exclude diseased myocardium or reshaping the ventricle to its normal elliptical shape. The primary goal is to decrease cavity size and wall stress, thereby enhancing cardiac efficiency.
BioVentrix’s Revivent TC System
Among the forefront is BioVentrix’s Revivent TC System, a transcatheter ventricular enhancement system and pioneering example of physical reverse remodeling devices. BioVentrix markets the Revivent TC System in Europe but the device is currently only approved for investigational use in the US.
The Revivent TC System is designed to address heart failure symptoms by excluding nonviable or scarred myocardium from the ventricular cavity. This technique is particularly beneficial for patients with significant ventricular scarring due to past myocardial infarctions, leading to abnormal chamber geometry and increased myocardial wall stress. The device effectively reshapes the left ventricle (LV) to decrease wall stress and improve heart function.
The Revivent TC system targets patients classified under New York Heart Association (NYHA) Class III-IV, who have experienced a myocardial infarction over 90 days prior, with significant LV dilation and viable myocardium opposite the scar. This minimally invasive procedure, requiring no sternotomy or cardiopulmonary bypass, demonstrates the system’s innovative approach to heart failure treatment.
The efficacy of the Revivent TC system is being evaluated through clinical trials, namely the LIVE trial and the ongoing ALIVE trial.
The LIVE trial showcased the system’s potential to reduce ventricular volumes by approximately 25 percent and improve left ventricular ejection fraction by about 16 percent. Additionally, patients experienced a notable increase in their six-minute walk distance, indicating improved physical capacity and quality of life. Survival rates were also impressive, exceeding 90 percent at one year.
The ALIVE trial, a pivotal study comparing the Revivent TC system with guideline-directed medical therapy (GDMT), aims to further establish the device’s efficacy and safety. With enrollment complete and follow-up ongoing, the results are pending, which could redefine treatment paradigms.
Ancora Heart’s AccuCinch Ventricular Restoration System
Another remarkable device in the reverse remodeling category is Ancora Heart’s AccuCinch Ventricular Restoration System. This transcatheter left ventricular restoration (TLVR) device is designed for heart failure patients who continue to experience symptoms despite standard medical treatment. In July 2022, the US Food and Drug Administration (FDA) granted Breakthrough Therapy designation to the AccuCinch device.
It works by placing and tightening anchors inside the LV to reduce its size, thus decreasing wall stress and improving heart failure symptoms. Located behind the mitral valve leaflets and chordae tendineae, the placement of the AccuCinch device is precisely guided by imaging techniques like transesophageal echocardiography (TEE) and fluoroscopy. The result is a significant reduction in left ventricular size, confirmed by angiograms and echocardiographic measurements after implantation.
The efficacy and safety of the AccuCinch device have been supported by pooled feasibility data from trial participants. The median implantation time is 131 minutes, with an average of 13 anchors placed per procedure. Importantly, the procedure has demonstrated a favorable safety profile, with no periprocedural deaths reported through one year of follow-up.
Patients have shown remarkable improvement in heart function and quality of life measures. Significant reductions in left ventricular end-diastolic volume (LV EDV) were maintained up to 12 months, alongside improvements in the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores and six-minute walk distances. These outcomes highlight the device’s potential in reversing heart failure progression and enhancing patient well-being.
Building on these promising results, the CORCINCH-HF trial represents the next step in evaluating the AccuCinch device. This randomized, open-label trial aims to enroll 400 patients across 80 sites, with a focus on assessing the device’s safety and efficacy. The primary efficacy endpoint is a hierarchical composite endpoint of all-cause deaths, left ventricular assist device (LVAD) implants or heart transplants. The primary safety endpoint is freedom from device- or femoral artery access-related major adverse events.
As the CORCINCH-HF trial progresses, the medical community anticipates further evaluation of the AccuCinch device’s role in transforming heart failure treatment.
Shunt Devices: Easing the Pressure
Moving beyond devices targeting the LV, there are also innovations in the form of shunt devices.
For example, interatrial shunt devices facilitate shunting from the high-pressure left atrium (LA) to the lower-pressure right atrium (RA), aiming to alleviate symptoms by reducing left atrial pressure. Elevated left atrial pressure — a central hallmark of heart failure — manifests as dyspnea (breathlessness) and can lead to progressive volume overload, significantly impairing the quality of life for those affected.
Corvia’s Interatrial Shunt Device
Among the devices that have undergone rigorous investigation, Corvia’s interatrial shunt device (IASD) stands out. This device was granted Breakthrough Device designation by the FDA in 2019.
Corvia’s IASD has been the subject of extensive study, particularly highlighted in the REDUCE LAP-HF 2 trial results presented in 2022. This trial explored the efficacy of the IASD in patients with heart failure with preserved ejection fraction (HFpEF) or heart failure with mid-range ejection fraction (HFmrEF). Unfortunately, the trial concluded that the IASD did not provide symptomatic or clinical benefits in the general patient population over a follow-up of two years.
However, a silver lining emerged with the indication that patients without latent pulmonary hypertension might benefit from this intervention, prompting further research in the ongoing RESPONDER-HF trial.
Edward’s APTURE Transcatheter Shunt System
Edwards Lifesciences’ APTURE transcatheter shunt system represents a new alternative, establishing a shunt from the LA to the coronary sinus, thus leveraging the natural blood flow path to the RA. This method preserves the intra-atrial septum for future interventions and potentially offers better accommodation of volume and pressure changes due to the compliance of the coronary sinus.
The APTURE device, still under investigation, aims to treat patients experiencing symptomatic heart failure characterized by increased left atrial pressure.
Initial outcomes from the ALT FLOW I trial, an open-label feasibility study of the device, showed promising results with a high success rate of implantation (90 percent). The feasibility data showed significant improvement in hemodynamic endpoints (at six months, there was a reduction in the 20-watt pulmonary capillary wedge pressure [PCWP] by 7 mm Hg compared to baseline) and an impressive boost in KCCQ scores (23-point mean improvement).
Building on these promising results, the APTURE shunt system is currently under further investigation in the ALT FLOW II trial. This prospective, multi-center, randomized sham-controlled trial aims to enroll 100 patients across 30 study sites, having begun enrollment in September 2023. With a focus on the safety of the shunt implant and its efficacy in reducing PCWP during exercise, this trial represents a critical step forward in validating this innovative treatment approach for heart failure.
Cardiovascular Neuromodulation for Combating Heart Failure
A promising avenue that has recently captured the attention of cardiologists worldwide is cardiovascular neuromodulation. This approach targets the autonomic nervous system’s dysregulation, a hallmark feature of heart failure, offering new hope for patients with this debilitating condition.
Heart failure is characterized by a chronic imbalance in the autonomic nervous system, leading to progressive maladaptation and adverse cardiovascular outcomes. Initially, this dysregulation acts as a compensatory mechanism to maintain blood pressure and cardiac output following cardiovascular insults, such as myocardial infarction. However, when unaddressed, it results in a vicious cycle of sympathetic reflex responses, triggering further contractile deterioration and favoring heart failure progression.
Recent advancements in autonomic regulation therapy have introduced electrical devices designed to restore the autonomic nervous system’s balance. By delivering electrical energy directly, these devices aim to promote vagal inhibitory control and dampen sympathetic drive, potentially arresting the progression of heart failure to some extent. Among these pioneering therapies, three modalities stand out: baroreceptor activation therapy (BAT), splanchnic nerve ablation and cardiac pulmonary nerve stimulation.
Baroreceptor Activation Therapy
BAT is a new approach in the management of heart failure. The therapy involves stimulating the carotid baroreceptors, sensory nerve endings located in the carotid artery walls. These receptors play a crucial role in regulating blood pressure by balancing sympathetic and parasympathetic activity. By activating these receptors, BAT aims to decrease heart rate, prevent pathological remodeling of the heart, increase vasodilation and reduce elevated blood pressure (Figure 1).
This minimally invasive procedure uses ultrasound imaging to precisely place an extravascular stimulation lead near the carotid baroreceptors, particularly around the carotid sinus. The efficacy of the stimulation can be quickly apparent during the procedure as it leads to a reduction in both blood pressure and heart rate.
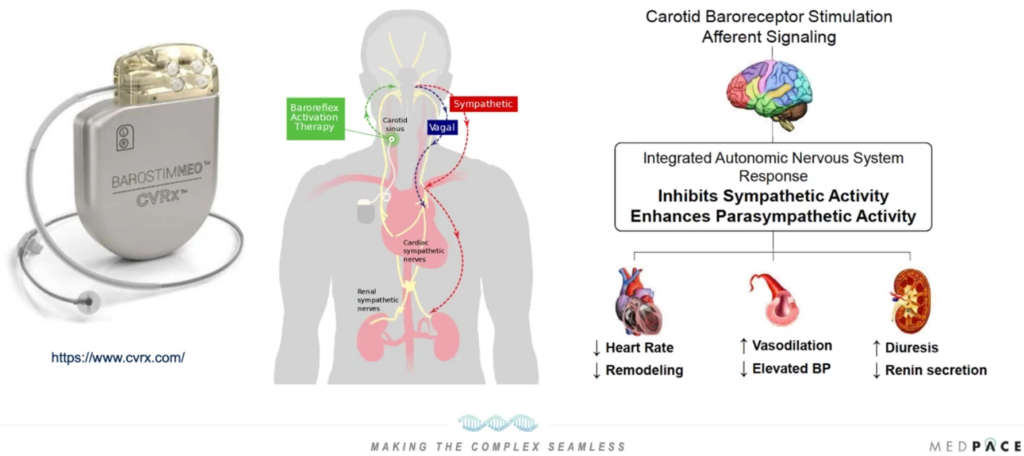
Figure 1. Baroreceptor activation therapy.
However, the application of BAT comes with specific contraindications. These include anatomical considerations like bilateral carotid bifurcations positioned above the mandible, conditions such as baroreflex failure or autonomic neuropathy, uncontrolled symptomatic cardiac bradyarrhythmias and substantial carotid atherosclerosis.
The foundational evidence supporting BAT’s efficacy emerges from the BeAT-HF trial, which explored its benefits in patients suffering from heart failure with reduced ejection fraction (HFrEF). CVRx’s Barostim NEO System was the BAT tested in the clinical study.
The six-month follow-up of cohort D within the BeAT-HF trial presented promising results. Significant improvements were noted in exercise capacity and quality of life, alongside favorable changes in biomarkers such as reductions in NT-proBNP levels. Despite the absence of data on long-term morbidity, mortality and cardiovascular structural changes, the trial laid the groundwork for further investigation.
The ongoing BiRD-HF trial in Germany promises to shed light on BAT’s potential to induce reverse cardiac remodeling in heart failure patients.
Following the promising outcomes of the initial research, CVRx’s BAT has carved a niche for itself in heart failure management. The Barostim NEO System received FDA approval in 2019 for use in specific HFrEF patients who have exhausted other treatment options. This approval has since been expanded, broadening the eligibility to include more patients based on clinical criteria, including NYHA Class and NT-proBNP levels.
BAT gives hope to those with heart failure, particularly when conventional treatments such as cardiac resynchronization therapy (CRT) are not viable options. Its minimally invasive nature and the extracardiac placement of the lead minimize the procedural risk, making it an attractive alternative for eligible patients.
Splanchnic Nerve Ablation
HFpEF presents a challenge in cardiovascular medicine, characterized by symptoms of heart failure despite a normal ejection fraction. Addressing this condition requires innovative treatments that go beyond traditional heart failure management strategies. One such innovative approach is splanchnic nerve ablation, a procedure designed to alleviate the symptoms of HFpEF by redistributing blood volume within the body.
The splanchnic nerve plays a crucial role in regulating blood flow to the liver and spleen, organs that together contain nearly a quarter of the body’s blood volume. In HFpEF patients, stimulating the greater splanchnic nerve increases cardiac preload and afterload, leading to elevated cardiopulmonary pressure. By ablating the greater splanchnic nerve, this procedure aims to reduce excessive splanchnic vasoconstriction, thereby facilitating the redistribution of blood volume. This can potentially alleviate the volume overload characteristic of HFpEF, improving patients’ symptoms and quality of life.
A first-in-human study of Axon Therapies’ investigational splanchnic nerve ablation system involved 11 patients with HFpEF, predominantly women, who exhibited NYHA Class II or III symptoms, had an ejection fraction greater than 50 percent and demonstrated elevated PCWP both at rest and during exercise. Implanted via the femoral vein, the device offered a novel approach to treating HFpEF. The study reported positive outcomes, including improved NYHA class, weight loss with a stable diuretic regimen and increased KCCQ scores.
The subsequent REBALANCE-HF study expanded on these findings, enrolling patients with similar criteria to the first-in-human study but incorporating a broader NYHA Class range and requiring a history of recent heart failure exacerbation or diuretic adjustment. Notably, the study included a sham group to provide a control.
While the REBALANCE-HF study confirmed the procedure’s safety, it found no significant differences in key indicators such as PCWP, right atrial pressure or pulmonary artery pressure between the treatment and sham groups. Similarly, there were no significant changes in KCCQ scores, six-minute walk test results or NT-proBNP levels.
Despite these overall findings, further analysis identified a subgroup of respondents who benefited from the procedure. These patients had preserved cardiac output during exercise or standing, no chronotropic insufficiency and no evidence of advanced structural or restrictive heart disease. Among these respondents, significant improvements were noted in KCCQ scores, six-minute walk test results and NT-proBNP levels. This finding has paved the way for further research to confirm these benefits and potentially refine patient eligibility criteria for this intervention.
Cardiac Pulmonary Nerve Stimulation
Cardiac pulmonary nerve stimulation (CPNS) is the latest advancement in neuromodulation techniques which is being investigated as an intervention in acute decompensatory heart failure.
The underlying mechanism of CPNS involves the autonomic nerve fibers that envelop the pulmonary arteries, particularly around the right pulmonary artery. These fibers play a role in regulating cardiac lusitropy and inotropy, traversing the anterior and posterior surfaces of the right and main pulmonary arteries. Animal studies have shown that stimulating these nerve fibers proximate to the heart yields localized effects, sidestepping the systemic side effects commonly associated with pharmacological interventions, such as inotropes.
At the center of this new approach is Cardionomic’s investigational CPNS System, which uses a neuromodulation stimulation catheter (the CN2 catheter) for its application. Designed for percutaneous delivery via the internal jugular vein, the process employs standard right heart catheterization techniques coupled with fluoroscopic guidance to precisely navigate towards the right pulmonary artery. Once positioned, the catheter is deployed against the artery’s wall, initiating stimulation through a spectrum of programmable parameters tailored to the patient’s physiological response.
The device can remain in situ, providing therapeutic benefits for up to five days, thereby offering a critical solution in acute care scenarios, notably in cases of acute decompensated heart failure.
Currently, the efficacy and safety of this CPNS are being evaluated through the STOP-ADHF trial. This study juxtaposes Cardionomic’s CPNS System plus standard care versus standard care alone, targeting an enrollment of approximately 90 participants.
Eligible patients for the STOP-ADHF trial are those hospitalized with acute decompensated heart failure, characterized by an NT-proBNP level exceeding 2,000 pg/mL, an ejection fraction of 50 percent or lower and symptomatic fluid overload. Notably, individuals with cardiogenic shock, significant renal dysfunction (eGFR less than 25 mL) or those requiring inotropic or mechanical support are excluded from participation. The primary safety outcomes of this trial will be assessed at six months post-intervention, with the study currently in progress.
By leveraging neuromodulation to specifically target the autonomic nerves surrounding the pulmonary arteries, CPNS potentially offers an alternative to conventional therapies, potentially reducing reliance on pharmacological agents and their associated systemic effects.
Innovations in Interventional Technologies for Valvular Heart Disease
The growing prevalence of valvular heart diseases globally underscores a critical need for advanced interventional treatments. As the demographic landscape shifts towards an older population, the incidence of conditions such as aortic stenosis, mitral regurgitation and tricuspid regurgitation has seen a marked increase. This demographic change, coupled with the limitations of traditional surgical options for many patients, has increased the demand for less invasive, more sophisticated interventional approaches.
Transcatheter Aortic Valve Replacement (TAVR) for Aortic Valve Stenosis
The transcatheter aortic valve replacement (TAVR) technique has been at the forefront of transforming the treatment landscape for patients suffering from aortic valve stenosis — a condition characterized by calcified, thickened valve leaflets obstructing blood flow from the heart to the aorta, affecting about 12 percent of individuals over 75.
The journey of TAVR began as an alternative to surgical aortic valve replacement (SAVR), targeting patients deemed at high or prohibitive risk for open-heart surgery. The early success of TAVR paved the way for its quick adoption, leading to extensive research, clinical trials and technological refinements.
Large-scale randomized clinical trials have shown that, in low surgical risk patients, TAVR matched SAVR in terms of reducing mortality, disabling strokes and rehospitalizations for valve-related issues; in some cases, TAVR surpassed SAVR in terms of reducing mortality and disabling strokes. These findings have been key in expanding the indications for TAVR, making it an option for a broader patient demographic.
Technological advancements in TAVR devices and delivery systems have been central to its evolution (Figure 2). The move towards miniaturizing the technology and enhancing the precision of valve deployment has led to significantly better procedural outcomes.
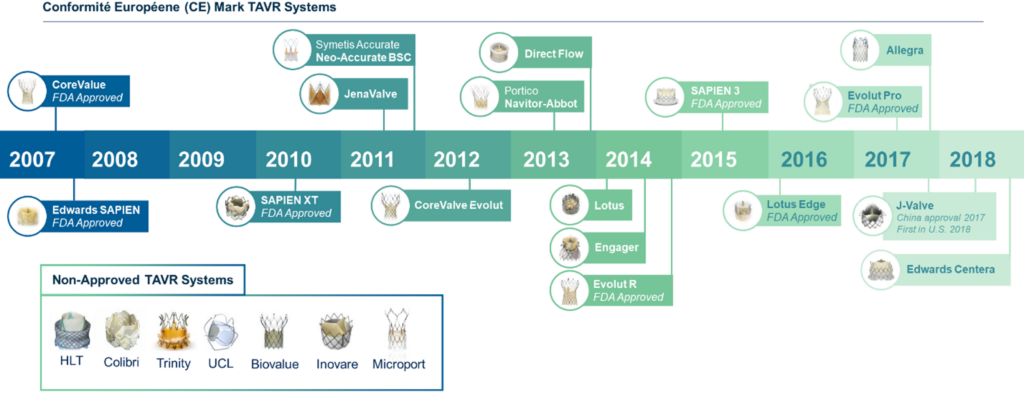
Figure 2. Transcatheter aortic valve replacement (TAVR) technology evolution.
Balloon-expandable transcatheter heart valves, like the experimental, artificial heart valve SAPIEN X4 from Edwards Lifesciences, have marked a substantial advancement in treating aortic valve stenosis. This catheter-based, minimally invasive technology eliminates the need for open-heart surgery or cardiopulmonary bypass. Since its initial clinical trial evaluation, the Edwards Lifesciences’ SAPIEN technology has evolved through four generations, focusing on enhanced safety and efficacy.
Addressing the Challenges of Aortic Valve Regurgitation
Aortic valve regurgitation, a condition characterized by the leaking of the aortic valve that allows blood to flow backward into the LV, presents unique challenges in the field of cardiac intervention.
The treatment of aortic valve regurgitation has traditionally been challenging due to several factors, including the variation in valve morphology and the absence of calcification, which plays a crucial role in anchoring transcatheter valves in cases of stenosis. Patients with aortic valve regurgitation often exhibit minimal or absent cusp calcification, dilated aortic roots and frequent coexistence of dilated ascending aorta, complicating the secure placement and sealing of the valve.
In response to these challenges, the development of specialized transcatheter aortic valves has been pivotal. Devices pending FDA approval like Genesis MedTech’s J-Valve Transfemoral (TF) System and JenaValve Technology’s Trilogy Transcatheter Heart Valve (THV) System have introduced innovative design features tailored to overcome the obstacles inherent in treating regurgitation.
For example, the J-Valve System uses unique anchoring mechanisms, resembling hula hoops, that facilitate accurate alignment and secure placement within the valve structure, even in the absence of calcification. These anchorings provide both mechanical stability and physiological benefits, such as facilitating coronary access and preventing thrombosis.
The effectiveness of these new devices is being rigorously tested in clinical trials. For instance, the ALIGN-AR trial for JenaValve, presented at the 2023 Transcatheter Cardiovascular Therapeutics (TCT) Annual Scientific Symposium and published in The Lancet, highlighted the device’s safety and efficacy in treating severe aortic regurgitation, meeting primary endpoints with statistical significance.
The technologic advancements provided by the J-Valve and Jena Valve appear to have addressed the technical challenges of suboptimal fluoroscopic visualization and the risk of device misplacement or migration present with currently available valves designed to treat aortic stenosis. Ongoing innovation and refinement of the transcatheter devices aim to enhance their visibility under imaging, improve their anchoring capabilities and reduce the risk of residual regurgitation.
Pioneering Mitral Valve Interventions for Mitral Valve Disease
The mitral valve’s complex anatomy and crucial role in heart function make managing its diseases, especially mitral regurgitation, challenging. The complexity of the mitral valve structure, including its leaflets, annulus, chordae tendineae and papillary muscles, demands a sophisticated approach to treatment.
Transcatheter mitral valve interventions (TMVI) have emerged, offering minimally invasive treatment options for patients unsuitable for conventional surgery.
Abbott’s FDA-approved MitraClip System, a pioneering technology for mitral regurgitation, exemplifies the advances in TMVI (Figure 3). This percutaneous technique, which involves clipping the mitral valve leaflets together to reduce regurgitation, has transformed the treatment landscape for patients at high surgical risk. Its success has paved the way for further innovations in mitral valve repair, including the development of other clip-based devices and techniques that offer improved precision and efficacy.
Innovative approaches to chordal modifications have also shown promise. Technologies like the NeoChord Artificial Chordae Delivery System and Edwards Lifesciences’ Harpoon System allow for the percutaneous replacement or repair of damaged chordae tendineae, addressing a common cause of mitral regurgitation (Figure 3).
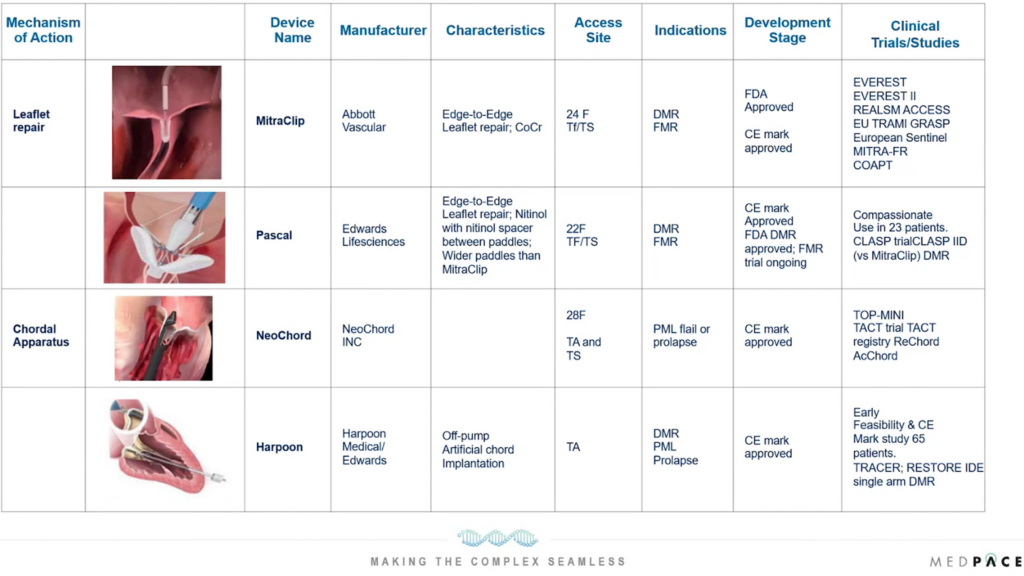
Figure 3. Innovative approaches to treat mitral regurgitation by targeting leaflet repair and the chordal apparatus.
Similarly, annuloplasty techniques, which aim to reshape or stabilize the mitral annulus to treat mitral valve dysfunction, have seen advancements with devices like Edwards Lifesciences’ Cardioband system and Mitralign Inc’s Mitralign system (Figure 4). The Carillon device, developed by Cardiac Dimensions Inc., offers a less direct method for annuloplasty by accessing the mitral annulus through the coronary sinus. This innovative approach is currently being tested in a pivotal trial, EMPOWER, for FDA approval.
 Figure 4. Annuloplasty techniques for treating mitral valve disease.
Figure 4. Annuloplasty techniques for treating mitral valve disease.
On the other hand, transcatheter mitral valve replacement (TMVR) addresses cases where repair is insufficient or not feasible, offering a complete valve replacement option without the need for open-heart surgery. Abbott Vascular’s Tendyne TMVR system is the only device with CE mark approval to date and is at the forefront of this technology (Figure 5).
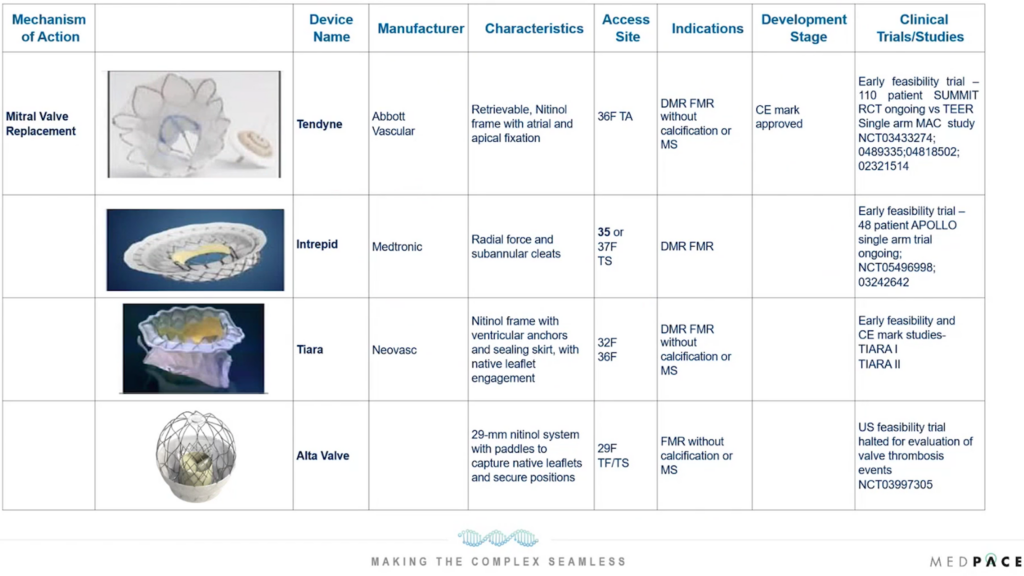 Figure 5. Innovative transcatheter mitral valve replacement (TMVR) technologies for the treatment of mitral valve disease.
Figure 5. Innovative transcatheter mitral valve replacement (TMVR) technologies for the treatment of mitral valve disease.
Despite the promise of TMVI, challenges remain, including the need for precise device placement, the risk of left ventricular outflow tract obstruction and ensuring long-term durability and efficacy. Ongoing research and clinical trials continue to refine these technologies, with the goal of expanding their applicability and improving patient outcomes.
Pioneering Tricuspid Valve Interventions for Tricuspid Valve Disease
Tricuspid valve disease, particularly tricuspid regurgitation, was often overlooked compared to left-sided heart valve diseases like mitral valve disease, but its importance for patient outcomes has led to innovations in tricuspid valve interventions (TVI).
Tricuspid regurgitation occurs when the tricuspid valve fails to close properly, allowing blood to flow backward from the right ventricle (RV) to the RA. This condition can lead to heart enlargement, heart failure and other serious complications. Traditional treatments were mainly surgical, restricted by high risks especially in patients with other health issues.
Coaptation devices, such as Abbott’s TriClip and Edwards Lifesciences’ Pascal systems, represent a significant breakthrough in TVI. These devices are designed to reduce tricuspid regurgitation by clipping together parts of the leaflets to improve valve closure.
Annuloplasty devices focus on addressing the dilatation of the tricuspid annulus, a common cause of tricuspid regurgitation. Technologies like Edwards Lifesciences’ Cardioband system use a catheter to place a series of sutureless anchors around the annulus, which are then cinched together to reduce its size and enhance valve coaptation. This approach mirrors techniques used in surgical annuloplasty but without the need for open-heart surgery, presenting a less invasive alternative.
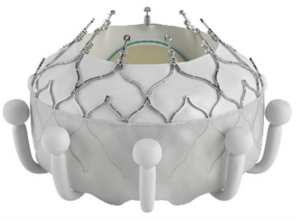
transcatheter tricuspid valve replacement system.
Photo courtesy of Edwards Lifesciences.
Perhaps the most ambitious advancement in TVI is the development of transcatheter tricuspid valve replacement (TTVR) technologies. In February 2024, Edwards Lifesciences’ EVOQUE tricuspid valve replacement system became the first transcatheter therapy to get FDA approval for the treatment of tricuspid regurgitation (Figure 6).
Devices like the EVOQUE TTVR system are pioneering this space, offering a complete valve replacement option for patients with severe tricuspid regurgitation.
Despite these advancements, TVI faces unique challenges, including the complex anatomy of the tricuspid valve, the risk of device-related complications and the need for precise imaging and procedural guidance. Ongoing research and clinical trials are essential to overcome these hurdles, refine these innovative technologies and broaden their applicability.
The innovative devices discussed by Dr. Lubert, Dr. Myat and Dr. Kereiakes mark a substantial shift in heart failure treatment paradigms and aim to address the mechanical aspects of the disease. As these trials progress, the potential for these technologies to improve patient outcomes and quality of life is immense, bringing in a new era in the management of heart failure.
To learn more about cardiovascular device development in heart failure, be sure to watch the on-demand webinar featuring the experts from Medpace.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.












Join or login to leave a comment
JOIN LOGIN