SurGenTec has received expanded 510(k) clearance from the FDA for its synthetic bone graft device, OsteoFlo HydroFiber. The device was first cleared in January 2025 as a stand-alone synthetic bone graft for spinal fusion procedures. The FDA considered it substantially equivalent to autograft.
The updated clearance broadens its use beyond spinal fusion. The material can now be used to fill bone voids or gaps from trauma, cysts, tumors or osteomyelitis — a bone infection that affects approximately 22 people per 100,000 annually in the US and often requires removal of the infected bone or tissue.
Bone voids are empty spaces in bone that result from injury, infection or surgery. If left unfilled, these gaps can disrupt healing or weaken the bone’s structure. They can occur in different surgical situations, such as removing tumors, treating bone infections or dealing with serious orthopedic injuries.
Traditionally, bone voids are treated using autografts — bone harvested from the patient — or allografts from donors. While effective, autografts require a secondary surgical site and come with risks of pain and complications. Allografts can vary in quality and have a minimal risk of disease transmission.
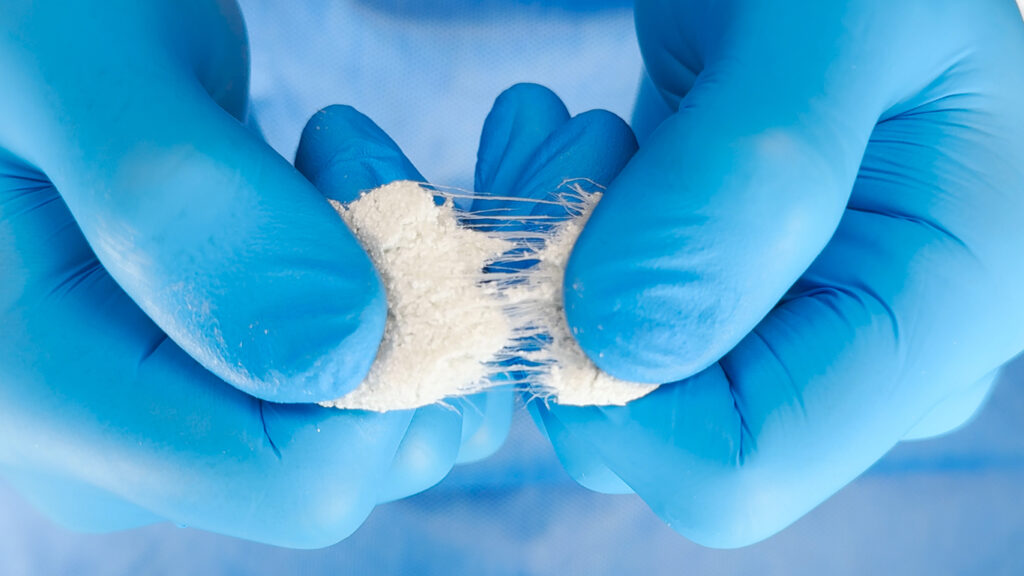
OsteoFlo HydroFiber offers an off-the-shelf synthetic alternative to these biologic materials. The graft contains bioactive synthetic granules suspended in a hydrophilic fiber matrix — which is to say, bone-like particles are held in a soft, absorbent fiber mesh that helps keep the material in place while supporting new bone growth.
This structure incorporates SurGenTec’s Web Interlace Technology, which stabilizes the granules and prevents them from shifting after implantation. When hydrated with saline, bone marrow aspirate or blood, the material becomes moldable and flowable, allowing surgeons to shape and position it precisely within a defect.
Surgeons deliver the graft using SurGenTec’s GraftGun, a system that places the material exactly where it’s needed, even in small or hard-to-reach areas during surgery. Once implanted, OsteoFlo HydroFiber serves as an osteoconductive scaffold that supports bone regeneration.
Unlike autografts, which require harvesting bone from another part of the body, OsteoFlo HydroFiber does not involve a second surgical procedure. Its design also helps address donor tissue variability, while allowing the material to conform to complex bone shapes and remain stable during healing without extra support.
OsteoFlo HydroFiber has gained attention at spine technology forums and is being increasingly adopted by spine surgeons. The company reports rising interest in the product because of its handling qualities and its function as a synthetic autograft substitute in difficult surgical sites.
What Else Is Happening in the Bone Graft Market?
The bone graft and substitutes market may reach $5.7 billion globally by 2035, driven by aging and a rising demand for spinal and dental procedures.
Here are some more developments from this year that point to ongoing innovation in bone grafts:
- Sanara MedTech got US rights to OsStic, a synthetic adhesive bone void filler designated as a Breakthrough Device. It’s made for fracture repair and offers a non-traditional approach to stabilizing bone segments.
- Evergen, in partnership with the University of Florida, launched an AI-powered image processing tool that can analyze bone graft CT scans within seconds. The tool will digitally evaluate graft integrity and placement more quickly.
- Elute FDA-cleared BonVie+, a resorbable calcium-based bone void filler that gradually dissolves and is replaced by the patient’s own bone, offering controlled remodeling in a variety of orthopedic cases.
- CGBio, a South Korea-based company, propelled its NOVOSIS PUTTY into US clinical trials after receiving an investigational device exemption (IDE) approval. The putty incorporates rhBMP-2, a lab-produced growth factor, to support bone formation in spinal fusion surgeries.
- Cerapedics gained FDA premarket approval for PearlMatrix P-15, a peptide-enhanced bone graft associated with faster lumbar fusion outcomes compared to autograft. The first US patient was treated in July 2025 following approval.
If you want your company to be featured on Xtalks.com, please email [email protected].











Join or login to leave a comment
JOIN LOGIN