Regulatory affairs professionals play a pivotal role in the healthcare, pharma and medical device industries, ensuring that products meet rigorous regulatory standards before reaching the market.
The 2024 Global Regulatory Affairs Professionals Workforce report reveals that nearly 125,000 professionals worldwide work in this field, with the highest concentrations in Europe, North America and Asia.
Salaries for regulatory professionals vary across industries, with biotech and pharma often offering the highest compensation, followed by medical devices and diagnostics.
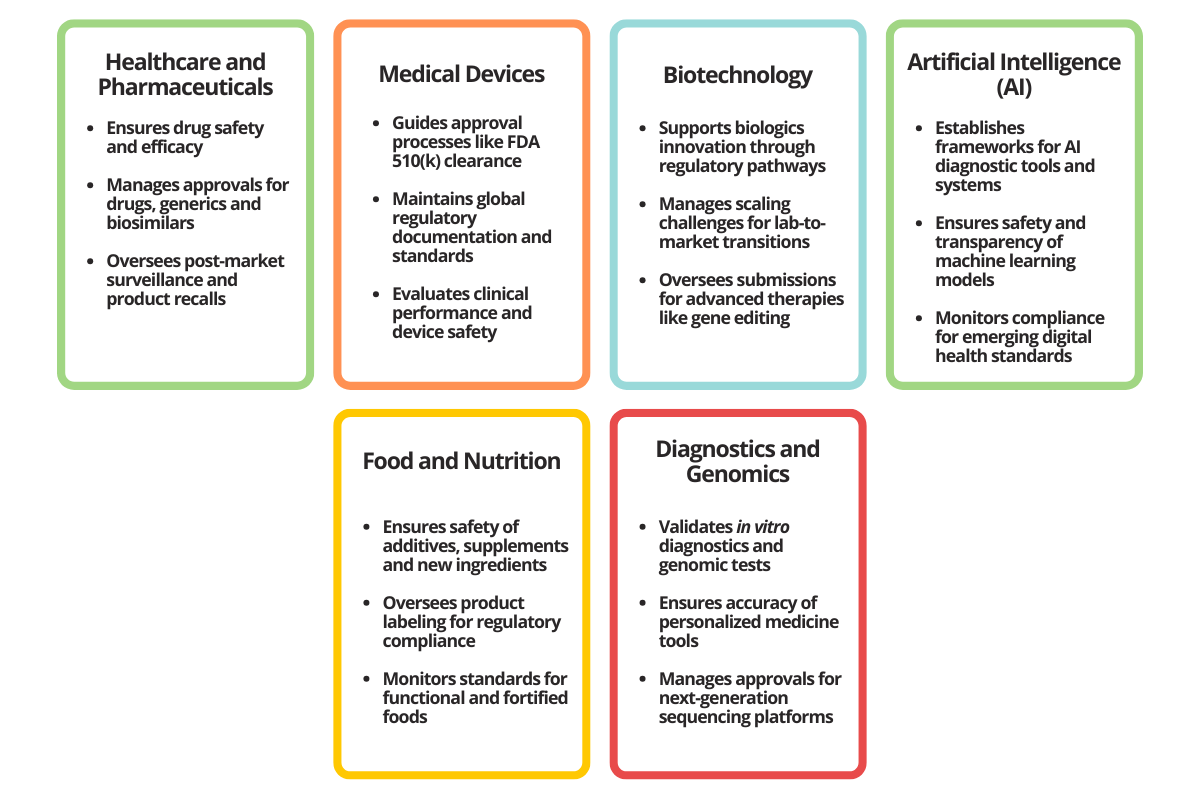
Regulatory affairs professionals play niche roles across the life sciences (Figure 1), such as navigating accelerated approvals for breakthrough drugs, ensuring compliance for innovative therapies and managing approvals for complex technologies like robotic surgical systems.
In 2024, regulatory affairs are advancing with the integration of artificial intelligence (AI) and machine learning (ML), which streamline data analysis and enhance pharmacovigilance processes. Generative AI is transforming regulatory operations, while AI-powered tools improve efficiency in submissions and decision-making. Projects like Orbis and the ACCESS Consortium exemplify how global harmonization is accelerating drug approvals and creating consistent frameworks for emerging therapies.
With rapid technological advancements and collaborative global initiatives, the field is poised for extraordinary growth. In this blog, explore the key roles in regulatory affairs, their responsibilities, required qualifications and how they contribute to advancing healthcare innovation. Be sure to explore the Xtalks Job Search platform to start your journey toward landing your dream regulatory affairs job in the life sciences.
Regulatory Affairs Associate
A Day in the Life
Regulatory Affairs Associates assist with document preparation, maintain compliance records and support submission processes. This entry-level position is foundational for building a career in regulatory affairs. Associates may work on projects like preparing technical files for product registration or managing correspondence with regulatory agencies.
Background, Skills and Know-How
A bachelor’s degree in life sciences is generally required. Many professionals in this role transition from positions such as research assistants or quality technicians.
Potential Pay
The typical salary range is $70,000 to $90,000 annually.
How to Approach the Regulatory Affairs Associate Role
Engaging in internships, online courses or quality assurance (QA) roles can provide a strong start in this field. Organizational skills and attention to detail are key traits in this position. Entry points often depend on adaptability and a keen interest in regulatory processes.
Regulatory Affairs Specialist
A Day in the Life
Regulatory Affairs Specialists spend their days preparing submissions, ensuring compliance and managing documentation to ensure adherence to US Food and Drug Administration (FDA) and international regulatory guidelines. They collaborate with cross-functional teams to address regulatory questions and maintain product approvals.
Background, Skills and Know-How
A bachelor’s degree in life sciences, engineering or a related field is generally required, along with one to three years of regulatory or QA experience. Many professionals in this role have academic or professional backgrounds in biology, chemistry or biomedical engineering.
Potential Pay
The typical annual salary ranges between $78,000 and $110,000.
How to Approach the Regulatory Affairs Specialist Role
Focus on certifications like Regulatory Affairs Certification (RAC) and gain experience in clinical or quality roles. Networking and specialized training enhance the chances of securing this role.
Pursuing certifications and transitioning from QA or clinical research roles can provide an excellent foundation.
Regulatory Affairs Manager
A Day in the Life
Regulatory Affairs Managers lead compliance strategies, act as liaisons with agencies and oversee international approval processes. Managers might lead projects like securing European CE Mark approval for a new pharmaceutical or updating compliance strategies following regulatory changes.
Background, Skills and Know-How
Candidates typically hold a bachelor’s or master’s degree in life sciences and possess five to eight years of regulatory experience. Expertise in project management and prior work in pharmaceuticals or medical devices are highly valued.
Potential Pay
Annual compensation typically falls between $110,000 and $150,000.
How to Approach the Regulatory Affairs Manager Role
Progression from a Regulatory Affairs Specialist role, coupled with leadership development and certifications like the Project Management Professional (PMP), can provide the necessary qualifications for this position. Gaining leadership experience and familiarity with global regulatory processes may further strengthen a candidate’s readiness.
Regulatory Affairs Director
A Day in the Life
Regulatory Affairs Directors lead teams, ensure global compliance and facilitate market access. Directors are often involved in strategic initiatives such as ensuring accelerated approval for groundbreaking therapies or managing compliance across multiple international markets.
Background, Skills and Know-How
This role often requires a master’s or PhD in life sciences or regulatory science, along with over 10 years of leadership experience. Professionals in this role often come from senior management positions in pharmaceuticals or biologics.
Potential Pay
Annual salaries for this role generally range from $150,000 to $200,000 or more.
How to Approach the Regulatory Affairs Director Role
Building expertise in strategic planning, global submission management and leadership is essential for this role. Advanced certifications and degrees can enhance qualifications, but diverse experiences in high-level decision-making are often equally valuable.
Regulatory Affairs Consultant
A Day in the Life
Regulatory Affairs Consultants offer specialized expertise to advise clients on regulatory pathways and develop compliance strategies. For instance, consultants may help a startup design regulatory strategies for a novel therapeutic or troubleshoot compliance issues for established companies.
Background, Skills and Know-How
Most consultants hold advanced degrees in life sciences or regulatory sciences and have five to 15 years of experience. Expertise in specific regulatory areas enhances the value consultants bring to their clients.
Potential Pay
Annual compensation typically ranges from $90,000 to $150,000, depending on expertise and project scope.
How to Approach the Regulatory Affairs Consultant Role
Establishing credibility through niche expertise and a strong professional network is a key component of becoming a consultant.
Regulatory affairs is transforming alongside the life sciences industry, driven by digital innovation and global collaboration. To navigate this evolving landscape, regulatory professionals need more than traditional expertise.
Digital literacy, such as understanding AI applications in compliance and regulatory frameworks, is becoming essential. As regulatory science becomes increasingly intertwined with innovation, professionals who embrace change and upskill are well-positioned to lead this transformation.
Start applying for your ideal job today. Visit Xtalks Job Search to see new and exciting openings in the pharma, biotech and medical device industries.












Join or login to leave a comment
JOIN LOGIN