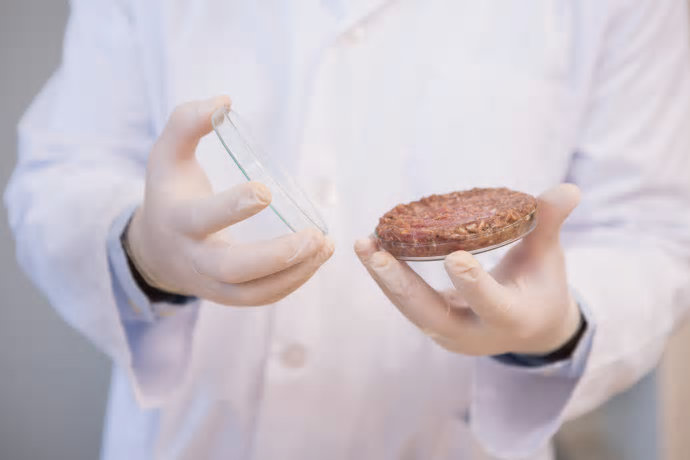
The two biggest regulatory bodies in the food industry are partnering to host a public meeting on a controversial food product: cell cultured meat. On Monday, US Secretary of Agriculture Sonny Perdue, and FDA Commissioner Scott Gottlieb announced that the joint public meeting will be held on October 23-24, 2018 to discuss the use of cell cultured technology to produce cultured meat.
This meeting will highlight the potential hazards associated with the production of lab-grown meat, oversight considerations and the labeling of cell cultured meat products in the food space.
“This is an important opportunity to hear from the agricultural industry and consumers as we consider the regulatory framework for these new products,” said Secretary Perdue. “American farmers and ranchers feed the world, but as technology advances, we must consider how to inspect and regulate to ensure food safety, regardless of the production method.”
“The FDA knows just how vital it is to ensure the safety of our nation’s food supply and the critical role science-based, modern regulatory frameworks are to fostering innovation. Recent advances in animal cell cultured food products present many important and timely technical and regulatory considerations for the FDA and our partners at USDA,” said Commissioner Gottlieb. “We look forward to the opportunity to hold a meeting with our USDA colleagues as part of an open public dialogue regarding these products.”
According to the two associations, the first day of the meeting will focus on potential hazards that need to be prevented and controlled during the production of cell cultured meat products. The regulatory bodies will also discuss oversight considerations during the first day of the meeting. The second day will be spent discussing labeling considerations.
The FDA and USDA are inviting representatives from the cell-cultured meat industry, the conventional meat industry, consumer groups and other stakeholders to attend the meeting. All attendees are requested to register on the Meetings and Events page which is on the FSIS website.
The two organizations are also welcoming written comments from the public regarding this matter. These comments can be submitted on regulations.gov by November 26, 2018.
This announcement comes after Memphis Meats, a cell-based meat producer, and the North American Meat Institute wrote a letter to President Trump in late August. The letter requested the government to divide the regulation of lab-grown meat between both the USDA and FDA. This might be why both regulatory organizations are working together to host this public meeting.
Although the North American Meat Institute seems to be coming to a truce with the lab-grown meat industry, the US Cattlemen’s Association is still against labeling cell-cultured protein as “meat.” The organization has been petitioning against using the term since the beginning of the year due to the potential market threat that the cell-cultured meat industry poses for the conventional meat industry.
This concern is no surprise considering the fact that many consumers are now ethically conscious when it comes to selecting their food products. Sustainability and the ethical treatment of animals have become top priorities for the majority of millennial consumers who are willing to pay more for these qualities. In fact, 70 percent of food companies that have animal welfare certified products have seen an increase in sales. This means that once cell cultured meat hits the market it has a lot of potential to attract ethical consumers who believe that lab-grown meat is better for the welfare of animals.
However, several regulatory decisions still need to be made before cell-cultured products hit grocery shelves. It will be interesting to see what the regulatory framework surrounding the lab-grown meat industry will look like once it hits the mainstream market.









Join or login to leave a comment
JOIN LOGIN