With North America accounting for nearly half of global contract manufacturing spend — and technologies like microfluidics and AI reshaping diagnostic production — a strategic approach to manufacturability is essential.
As demand grows for rapid, decentralized testing, early-stage collaboration between diagnostic developers and manufacturers has become necessary.
When diagnostic technologies stall at the scaling phase, it’s rarely due to a lack of innovation. More often, it’s because manufacturability wasn’t considered early enough — or deeply enough — in the development cycle.

CEO & Co-Founder
AmplifiDx

President & CEO
Drummond Scientific Company
In this Xtalks Spotlight interview, Dr. Nancy Schoenbrunner, CEO and Co-Founder of AmplifiDx, and Dr. Chris Strohsahl, President and CEO of Drummond Scientific Company, discuss how an early manufacturing partnership shaped the development of AmplifiDx’s DX 100 system and created space for iterative design that supported both clinical and commercial goals.
Why Drummond Chose AmplifiDx

When asked what led Drummond Scientific to partner with AmplifiDx towards the development process for the DX 100 system (Figure 1), Dr. Strohsahl explained that their process is similar to that of an investor conducting due diligence.
“When we decide to work with companies, we oftentimes view it as a professional investor would and we go through a diligence process to vet the opportunity in general,” he explained.
“Does it fit with our mission? Is it a company that’s developing something that either creates lives or helps to save lives? Is it addressing a market need, both in terms of a disease state that’s prevalent and large? Is there a reimbursement strategy for the device that they’re going down?”
Dr. Strohsahl said that beyond technical fit, what stood out was Dr. Schoenbrunner’s strong track record and the positive working relationship they’ve built over time.
Why AmplifiDx Brought in a Manufacturer Early
Dr. Schoenbrunner, for her part, emphasized that Drummond’s collaborative mindset — particularly their openness to shared development — made them stand out from other manufacturers.
“It comes down to capabilities, willingness, and attitude, and Drummond especially, they have this unique venture manufacturing philosophy, and that’s very unique amongst contract manufacturers,” she said.
She explained that while Drummond had the engineering strength she was looking for, the real appeal was their openness to co-develop. Unlike firms that push products into predefined workflows, Drummond allowed room for flexibility, even if not all their capabilities were in place at the start.
Dr. Schoenbrunner added that working with Drummond has supported a collaborative development environment, grounded in mutual respect and shared goals.
What Collaboration Looks Like in Practice
According to Dr. Schoenbrunner, the partnership is maintained through consistent check-ins that reflect the phase of development, along with hands-on feedback that directly informs design decisions.
“On the logistics side, we meet regularly, depending on how intense the phase is, it could be weekly, it could be biweekly, monthly if things are a little slow, just to make sure that we can get cartridges that are well made by Drummond for important field studies or clinical studies,” she explained.
“As we’ve iterated on the design of the DX 100 cartridge [Figure 2], we’ve included Drummond in our design reviews. We’ll get their feedback on different aspects to make sure that it will be manufacturable for them.”
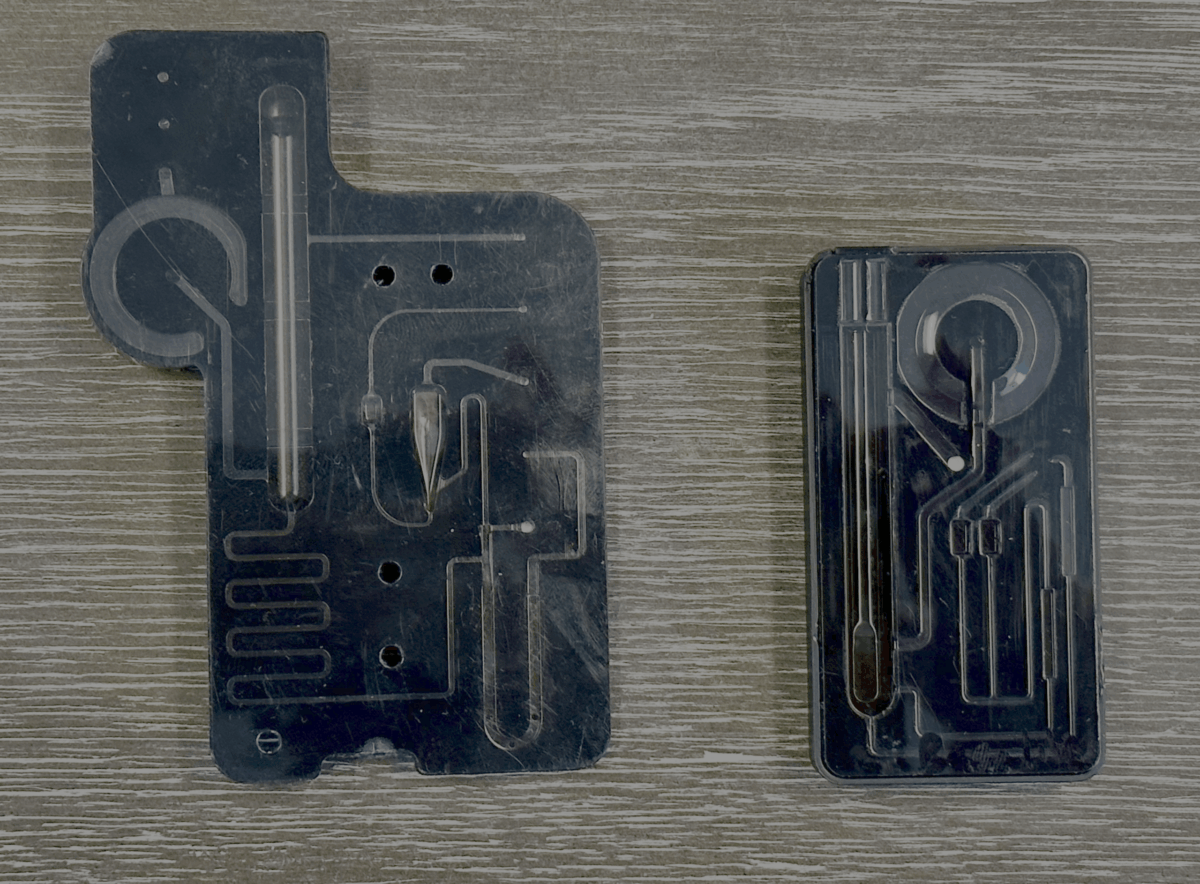
The design collaboration touched on multiple areas, including automating film assembly on top of the cartridge and removing a gasket from the initial version to streamline production.
Dr. Schoenbrunner noted that some of these improvements emerged from conversations spanning months, even years, and were reinforced through frequent design reviews.
Why Many Diagnostics Companies Struggle with Manufacturability
Dr. Strohsahl noted that a common hurdle for diagnostics startups is the late-stage realization that scaling wasn’t built into the original plan.
“The real answer is they just don’t think about it in time, and that’s not their fault,” he said.
He explained that designing assays that work in clinical settings is inherently difficult. Many scientists hesitate to layer on what they see as constraints during the innovation phase, which can delay critical manufacturability considerations.
“And this is where you get into the problems… [i.e. creating a device that gives the desired clinical performance]… but are you building a bridge to nowhere if it’s not manufacturable?”
He emphasized the importance of engaging manufacturers at the earliest stages — even while the assay chemistry is still being finalized — to ensure that design decisions support eventual mass production.
Advice to Other Diagnostics Startups: Keep It Simple, Start Early
Both speakers shared lessons for other diagnostics startups looking to bridge the gap between R&D and commercial viability.
“Start early. Start early, even if you maybe don’t have extensive financial resources, well, you want to pick your partner wisely. A partner that’s going to be willing to at least invest some time in you early to give you that feedback,” said Dr. Schoenbrunner.
“It doesn’t need to have been as intense as what we’re going through, which is wonderful. It’s such a gift, but at least being able to speak with somebody that’s on the manufacturing side early,” she added.
Dr. Schoenbrunner said their team focused on simplifying the chemistry from the outset, with the understanding that streamlined chemistry could support a simpler cartridge design and a more cost-effective instrument.
Dr. Strohsahl added that this shift toward simplicity enabled Drummond to manufacture at scale without compromising performance — a crucial step in achieving scalable production.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN