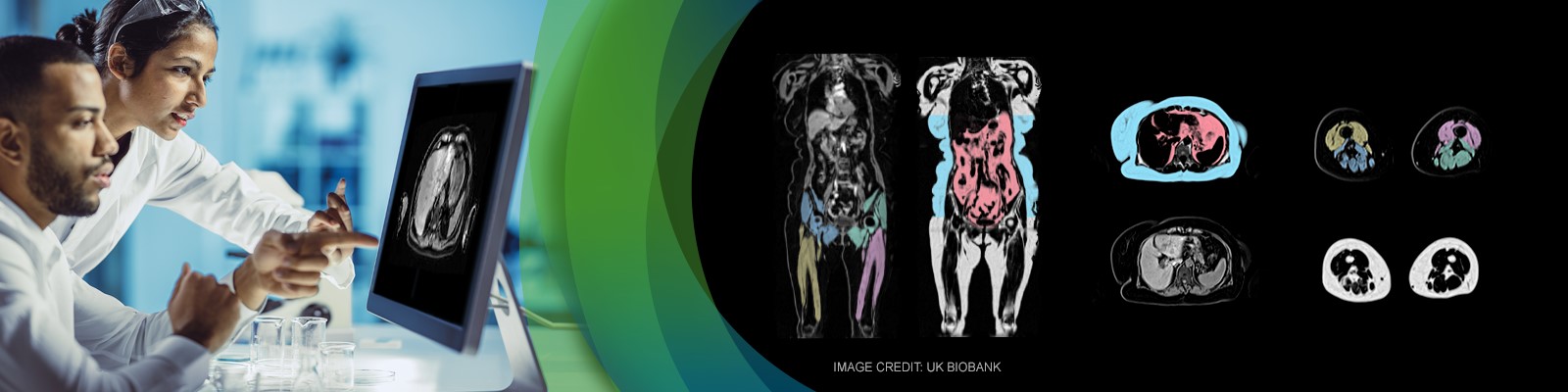
According to the WHO, obesity has reached epidemic proportions globally, with at least 2.8 million people dying each year as a result of being overweight or obese. The number of obese children and adolescents (aged five to 19 years) worldwide has risen tenfold in the past four decades. Obesity is a major risk factor for heart disease, stroke, osteoarthritis, diabetes, liver disease such as non-alcoholic steatohepatitis (NASH), and certain cancers. Muscular dystrophies, such as Duchenne, myotonic, facioscapulohumeral, etc., currently have no cure. Staging and progression of these conditions can be measured by the amount of muscle tissue that has been replaced by fat. As pharmaceutical companies attempt to develop treatments for these noncommunicable, obesity-related diseases and for the different muscular dystrophies, it is important to monitor body composition as both an efficacy and safety endpoint. The traditional body composition measurement has been BMI. Although BMI is a rough estimate of how over- or underweight a person is, it does not provide information about how much of the weight comes from muscle, how much comes from fat and how these tissues are affected by the treatment.
Join BioTel Research’s Vice President of Musculoskeletal and Metabolic Imaging, Jonathan Riek, PhD, and AMRA’s Chief Scientific Officer & Co-Founder, Olof Dahlqvist Leinhard, PhD, as they present alternative, noninvasive imaging methods to determine body composition. These imaging methods range from the most simplistic thickness measurements from ultrasound, to the more advanced measurements of fat distribution, muscle tissue, and diffuse infiltration of fat in muscle and liver tissue from DXA, CT and MRI.
The speakers will discuss:
- Advantages of noninvasive imaging methods
- When to use ultrasound, DXA, CT and MRI to measure body composition and how to successfully implement these modalities in clinical trials
- Body composition measurements from rapid, highly standardized, whole-body MRI
Register today to learn about the alternative, noninvasive imaging methods available to determine body composition in your clinical trial.
Speakers

Jonathan Riek, PhD, Vice President, Musculoskeletal and Metabolic Imaging, Philips Pharma Solutions
Jonathan Riek, PhD, serves as the Vice President of Musculoskeletal & Metabolic Imaging for BioTel Research. He has a PhD in Electrical Engineering from the University of Rochester, and his doctoral thesis was on motion and artifact reduction in MRI. He worked on the development of a 3D CT scanner as a postdoctoral researcher and then spent 7 years in the research labs at the Eastman Kodak Company. While at Kodak, Jonathan worked in the areas of motion estimation, frame rate conversion and video compression. He also served as the liaison between the image science division and the corporate software group. He joined VirtualScopics in 2000 to return to medical imaging and apply his skills interfacing between image science and software. In 2004, he led the development of the system that VirtualScopics still uses to run clinical trials and analyze medical images. In his current role, Jonathan has scientific responsibility for all of BioTel Research’s musculoskeletal and metabolic imaging studies, including arthritis, muscle diseases, diabetes and liver diseases such as NASH and NAFLD. He holds four patents and has published many articles on various topics in MRI.

Olof Dahlqvist Leinhard, PhD, Chief Scientific Officer & Co-Founder, AMRA
Olof Dahlqvist Leinhard, PhD is the Chief Scientific Officer & Co-Founder at AMRA. He is also a Senior University Lecturer in Magnetic Resonance (MR) Physics at Linköping University (LiU), within the Department of Medicine and Health (IMH) / Division of Radiological Sciences (RAD). Renowned within the field of MR Physics, Olof has over 45 peer-reviewed journal and conference articles, as well as over 90 peer-reviewed conference abstracts to his name.
Who Should Attend?
This webinar will benefit medical and non-medical professionals in the biopharmaceutical industry, especially those supporting imaging drug development with roles in:
- Clinical Research
- Clinical Development
- Medical Affairs
- Clinical Operations
- Project Management
- Regulatory Affairs
- Procurement
- Outsourcing
Xtalks Partners
BioTel Research
As leaders in clinical trials services for many years, experts from Cardiocore and VirtualScopics are the Research division of BioTelemetry, Inc., one of the world’s largest connected health companies. As BioTel Research, they provide advanced imaging services in metabolic, oncology, cardiovascular, musculoskeletal, neurologic and medical device studies, as well as cardiovascular monitoring for all therapeutic areas. BioTel Research’s team is comprised of key opinion leaders, radiologists and board-certified cardiologists, sub-specialty scientists, and highly trained technicians — who acquire, evaluate, and report high-quality data through an efficient database infrastructure. For more information please visit www.gobio.com/clinical-research.
AMRA
AMRA is the first in the world to transform images from a 6-minute, whole-body MRI scan into multiple 3D-volumetric fat and muscle measurements via a cloud-based analysis service. AMRA’s “one-stop-shop” service offers imaging biomarkers that are validated for high accuracy and precision, standardized and reproducible across all major MR platforms, and that reduce the overall patient burden. This has far-reaching implications for clinical and academic research, supporting findings that can be applied to therapeutic product investigation and, soon, to patient management. For more information, visit www.amramedical.com.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account