The US Food and Drug Administration (FDA) recently approved Boston Scientific’s Agent drug-coated balloon (DCB) for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease (CAD). While this technology has received approval in other countries, it marks the first drug-coated coronary balloon to gain approval in the US. This development could significantly impact CAD management, particularly for patients suffering from ISR, a condition characterized by the re-narrowing of arteries previously treated with stents.
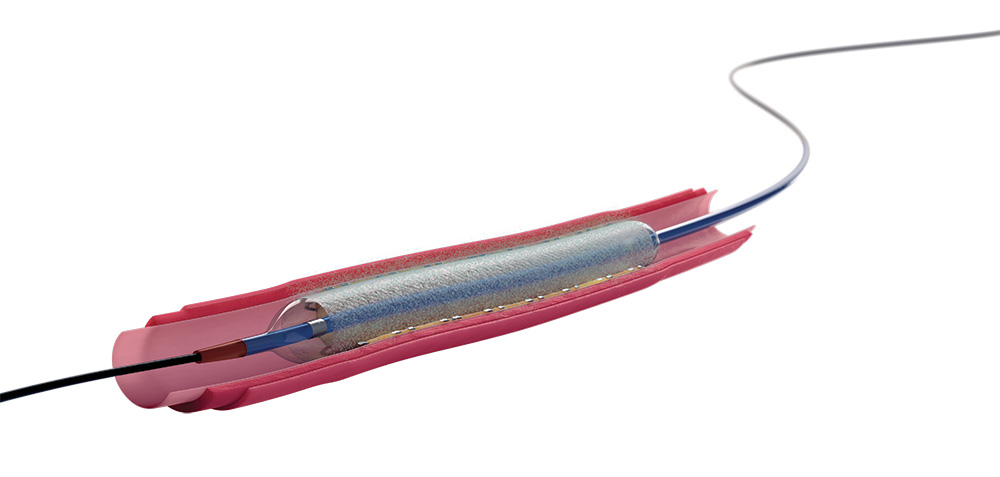
The Agent DCB delivers a paclitaxel-coated balloon catheter directly to the vessel wall, offering a novel therapeutic approach that can prevent the reoccurrence of ISR by transferring a therapeutic dose of paclitaxel.
The FDA’s approval is based on positive results from the AGENT IDE trial, a multicenter, prospective, randomized controlled study, which demonstrated the statistical superiority of Agent DCB over uncoated balloon angioplasty in preventing target lesion failure (TLF) at 12 months.
With over 100,000 patients already treated using this technology, its clinical efficacy and safety have been substantiated in both clinical and commercial settings. The positive AGENT IDE trial results also highlight the impressive safety profile of zero definite/probable cases of stent thrombosis and a considerable reduction in heart attack risk at the target vessel, providing a strong foundation for the adoption of Agent DCB.
XTALKS WEBINAR: Navigating Complex Regulations and Complaints to Achieve Quality Compliance for Life Sciences and Medical Devices
Live and On-Demand: Monday, June 24, 2024, at 2pm EDT (11am PDT)
Register for this free webinar to gain insights into leveraging an enterprise quality management system (EQMS) for best practices for navigating regulatory challenges and streamlining complaints management.
Key Features of the Agent Drug-Coated Balloon
One of the standout features of the Agent DCB is its TransPax Coating Technology. This proprietary technology ensures a uniform and controlled release of paclitaxel into the vessel wall during balloon inflation. This precise delivery mechanism is crucial for effectively preventing the reoccurrence of restenosis without compromising the integrity of the vessel.
The design of the Agent DCB focuses on optimal delivery. It offers a high degree of deliverability, conformability and rewrap, making it suitable for a wide range of lesion types, including those in challenging anatomical locations. This flexibility ensures that the Agent DCB can be effectively used in various clinical scenarios, providing physicians with a versatile tool for treating coronary artery disease.
The introduction of the Agent DCB offers a dedicated treatment option that avoids the need for additional stenting or the use of radiation. This is particularly beneficial for patients at high risk due to conditions such as diabetes or the presence of multi-layer stents. The Agent DCB provides a novel solution that can improve patient outcomes and enhance the overall management of ISR in CAD patients.
Current Treatment Strategies for Restenosis
Restenosis, the recurrence of stenosis, is the narrowing of a blood vessel leading to restricted blood flow. This often occurs after procedures such as percutaneous coronary intervention (PCI) with balloon angioplasty or the placement of coronary stents to open blocked arteries.
ISR is a specific type of restenosis occurring within a stent. It involves the growth of scar tissue in the artery or neointimal hyperplasia (the proliferation of smooth muscle cells in the stent), which can lead to artery narrowing.
Treatment options for restenosis focus on both mechanical and pharmacological interventions to reduce the possibility of recurrence and manage the condition if it reappears:
- Medication: To prevent blood clots in the artery, patients are prescribed medications such as aspirin and clopidogrel, which inhibit platelet aggregation. While these drugs do not directly prevent restenosis, they are crucial for preventing thrombosis, especially after stent placement.
- Repeat PCI: For patients experiencing restenosis, a repeat PCI procedure can be performed. This may involve using a balloon catheter to re-widen the artery (balloon angioplasty) or placing another stent (either bare-metal or drug-eluting) within the original stent to improve blood flow.
- Drug-eluting stents (DES): DES reduce the incidence of restenosis and are coated with drugs that inhibit scar tissue growth. Compared to bare-metal stents (BMS), DES significantly reduce restenosis rates.
- DCBs: DCBs, such as the Agent DCB, combine mechanical widening of the artery with drug delivery to the vessel wall to prevent cell proliferation. They are particularly useful for treating ISR because they do not leave any additional metal implant behind while directly delivering anti-proliferative medication to the affected area.
- Coronary artery bypass grafting (CABG): For cases of extensive or recurrent restenosis, especially in multiple arteries, CABG may be considered. This procedure creates a new pathway for blood flow to the heart by bypassing narrowed or blocked segments of coronary arteries.
- Radiation therapy (brachytherapy): This involves directly delivering radiation to the restenosis site to inhibit scar tissue growth. Although less common and typically reserved for cases where other treatment strategies have failed, brachytherapy has been effective for treating certain instances of ISR.
Market Dynamics: Understanding Agent DCB’s Competitors
Boston Scientific’s Agent DCB operates in an extremely competitive market. The introduction of Agent DCB offers an alternative to DES for certain patient groups, reflecting the ongoing trend toward developing more sophisticated, less invasive treatment options that provide lasting benefits with fewer complications. Many companies have already developed their own DCBs, each with unique features supported by varying levels of clinical evidence.
Medtronic’s IN.PACT Admiral DCB is used for treating peripheral artery disease (PAD), particularly in superficial femoral and popliteal arteries. Similar to the Agent DCB, it is coated with paclitaxel and leverages a proprietary coating technology that facilitates drug transfer and uptake.
B. Braun’s SeQuent Please NEO is designed for treating coronary artery lesions and uses a unique coating technology that includes paclitaxel and a carrier for enhanced drug delivery. It is used for patients with CAD, including those with de novo lesions, small vessel disease and ISR.
Cook Medical’s Zilver PTX, although primarily a DES for PAD, combines the mechanical support of a stent with local drug delivery to inhibit restenosis. This hybrid approach demonstrates the versatility and potential overlap in technology between DES and DCB markets.
Philips’ Stellarex DCB, designed for treating PAD, showcases Philips’ capability in the DCB market. Its technology and clinical success further emphasize the competitive landscape of PAD treatment.
The Agent DCB may be a game-changer in coronary care for healthcare professionals, particularly interventional cardiologists as it offers a new tool for addressing both CAD and ISR. Moreover, the Agent DCB enhances patient care by providing a treatment option that directly targets the mechanisms of restenosis, potentially leading to improved long-term outcomes for patients.
Moreover, patients benefit from a treatment option that reduces the likelihood of restenosis and repeat interventions, thereby improving their overall quality of life. The Agent DCB exemplifies the shift towards more personalized treatment strategies in CAD management, taking into account the patient’s specific condition and risk factors.
The introduction of the Agent DCB into the US market sets a new standard in ISR treatment and could potentially extend its use to other coronary conditions. Integrating the Agent DCB into clinical practice will rely on effective knowledge dissemination and training, as well as ongoing assessments of patient outcomes and broader evaluations of its efficacy and safety.
Furthermore, the Agent DCB’s availability in Europe, parts of Asia-Pacific and Latin America has already established its effectiveness and adaptability in treating ISR and previously untreated small vessel coronary disease. Treatment options like it not only have the potential to improve patient care but also to influence future research directions and treatment strategies in interventional cardiology.











Join or login to leave a comment
JOIN LOGIN