According to the National Institutes of Health (NIH), over 7,000 identified rare diseases affect an estimated 300 million people worldwide. In fact, the non-profit organization RareX released a report titled The Power of Being Counted in June 2022 that currently identifies the number of recognized rare diseases at 10,867. Despite their individual rarity, the cumulative impact of rare diseases is staggering, with approximately one in 20 people affected by a rare condition at some point in their lives.
Rare diseases, by their very nature, present unique challenges to patients, caregivers and healthcare professionals alike. Defined by their low prevalence, these conditions often elude diagnosis, lack effective treatments and impose significant burdens on those affected.
Orphan drug development poses many challenges that are unique to rare diseases, including recruitment in small patient populations, variable progression with heterogenous clinical manifestations and limited understanding of disease progression and natural history. Participant recruitment is challenging, especially in those indications in which there are competing clinical trials necessitating a comprehensive understanding of the disease research ‘landscape’ and targeted outreach and stakeholder collaboration. Designing trials with the most relevant endpoints can also be challenging without validated biomarkers or an adequate understanding of the natural history of the rare disease.
In recent years, rare disease research has shifted significantly, with patients playing an important and proactive role in clinical research. Partnership with patients is now fundamental to drug development, influencing clinical endpoints that are most relevant to patients, study designs and treatment strategies. This collaborative approach not only empowers patients but also raises awareness of the clinical trial within the patient community and so contributes to robust recruitment and success of clinical trials and treatments.
Recent breakthroughs in rare disease treatment showcase the power of innovation and collaboration between Sponsors, CROs and patients.
“One of the challenges in rare disease research is that patients are, by definition, rare and often spread across large geographical distances around the world. Consequently, the Sponsor and especially the CRO needs to have a global footprint to support patient participation in rare disease trials,” says Kristin Black, Clinical Trial Manager at Medpace.
Kristin Black recently spoke on a webinar where she and her colleague Miaesha Campbell, Senior Director of Patient Recruitment at Medpace, discussed how a patient-first approach addresses the unique challenges in rare disease research, drawing from their experiences with Pompe programs. They were joined by their colleague Dr. Terence Eagleton, MBBS, BSc (Hons), Senior Medical Director at Medpace who outlined some of the complexities of LOPD and the urgent need to develop new effective treatments.
Read on to learn key lessons in rare disease drug development.
Understanding Late-Onset Pompe Disease
Pompe disease, also known as glycogen storage disease type II (GSD2) or acid maltase deficiency, is a rare genetic disorder with an approximate incidence of one in 40,000 births characterized by the deficiency of the enzyme acid alpha-glucosidase (GAA). This enzyme deficiency leads to the accumulation of glycogen in various tissues, particularly muscles, impairing their function and resulting in progressive muscle weakness and wasting.
There are two distinct phenotypes of Pompe disease. The first is the infantile-onset form, which typically manifests within the first few months of life. This form progresses rapidly and is marked by severe symptoms such as generalized muscle weakness, cardiomyopathy and hypotonia, and often results in death within the first year of life due to respiratory failure.
The second phenotype, late-onset Pompe disease (LOPD), includes both juvenile- and adult-onset cases. LOPD can manifest as early as one year of age or as late as the sixth decade of life. Unlike the infantile form, LOPD presents with progressive skeletal muscle weakness and respiratory insufficiency. Without treatment, LOPD can eventually lead to respiratory failure, highlighting the importance of early intervention and management strategies.
Dr. Eagleton, Senior Medical Director at Medpace, explained that LOPD presents a unique challenge in clinical research due to its variable clinical presentation and unpredictable progression which, without due care, could introduce unwanted variables into the final dataset. While some individuals may experience mild symptoms and a slower disease course, others may face debilitating muscle weakness and respiratory complications.
The Importance of Avoiding Diagnostic Delays in LOPD
According to Dr. Eagleton, when suspecting LOPD, the diagnostic process typically involves a comprehensive assessment. This includes evaluating the patient’s family history of muscle disease, onset of symptoms and rate of disease progression.
Clinical evaluation of LOPD focuses on a neurological assessment, with a particular emphasis on progressive limb girdle weakness. Trunk muscle strength is evaluated using manual or quantitative assessments, such as the Muscle Research Council (MRC) scale, and basic function tests like Gower’s maneuver. During Gower’s maneuver, patients with LOPD use distinct muscle groups compared to typical individuals when transitioning from a supine to an upright position. Tongue weakness is also examined as an early sign of the disease.
Laboratory tests for LOPD detection, including blood biochemistry analysis, are done to assess markers such as creatinine kinase, troponin T, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Additionally, electromyography (EMG) may reveal a myopathic pattern, while spirometry measures respiratory function.
Muscle magnetic resonance imaging (MRI) has emerged as a valuable diagnostic tool, particularly for early detection. It reveals a typical pattern of axial muscle involvement and the presence of a ‘bright tongue sign,’ indicative of LOPD.
Dried blood spot (DBS) tests are increasingly used for diagnosis due to their specificity, sensitivity and rapid results, detecting reduced GAA enzyme activity in LOPD. Positive DBS results often prompt genetic studies to assess specific mutations, considered the gold standard for confirming LOPD diagnosis. Next-generation sequencing (NGS) allows for efficient analysis of known genetic mutations, enhancing diagnostic accuracy.
Overall, the diagnostic process for LOPD typically involves a series of assessments, including clinical, laboratory and imaging tests, culminating in genetic analysis to confirm the diagnosis. Dr. Eagleton highlights that confirmation through both enzymatic and genetic testing is preferred for a comprehensive diagnosis of LOPD.
Unfortunately, due to low disease awareness or lack of testing facilities, there can be a substantial delay in diagnosing LOPD, which can be up to eight to ten years.
“The prolonged diagnostic delay of LOPD is important because we know from natural history studies that disease severity increases with disease duration such that for every year from diagnosis without treatment, the odds for wheelchair use increase by 13 percent, and the odds for respiratory support increase by about eight percent,” explains Dr. Eagleton.
Due to the diagnostic challenges and delays associated with LOPD, it becomes imperative to explore effective treatment options, such as enzyme replacement therapies (ERTs).
Treatment with Enzyme Replacement Therapy
The management of LOPD requires a multidisciplinary approach due to its systemic nature. Since 2006, ERT has been the cornerstone of treatment for all Pompe disease patients. ERT involves intravenous administration of recombinant human acid alpha-glucosidase (rhGAA), replacing the deficient enzyme in patients with Pompe disease.
“Enzyme replacement therapy (ERT) has been shown to change the natural progression of LOPD for the better in most patients.”
— Terence Eagleton
“Studies of rhGAA have shown that ERT improves or stabilizes muscle strength, pulmonary function and other disease symptoms with increased survival in many patients,” says Dr. Eagleton.
However, historically, the therapeutic responses vary in magnitude among individual patients, highlighting an existing unmet medical need for new treatments.
Several ERTs are currently approved for the treatment of LOPD, including alglucosidase alfa (Myozyme/Lumizyme) and avalglucosidase alfa (Nexviazyme). Additionally, the combination therapy, cipaglucosidase alfa and miglustat (Pombiliti plus Opfolda), represents a significant milestone in managing LOPD (Figure 1). This innovative approach combines enzyme replacement with an enzyme stabilizer, offering new hope for patients with LOPD.
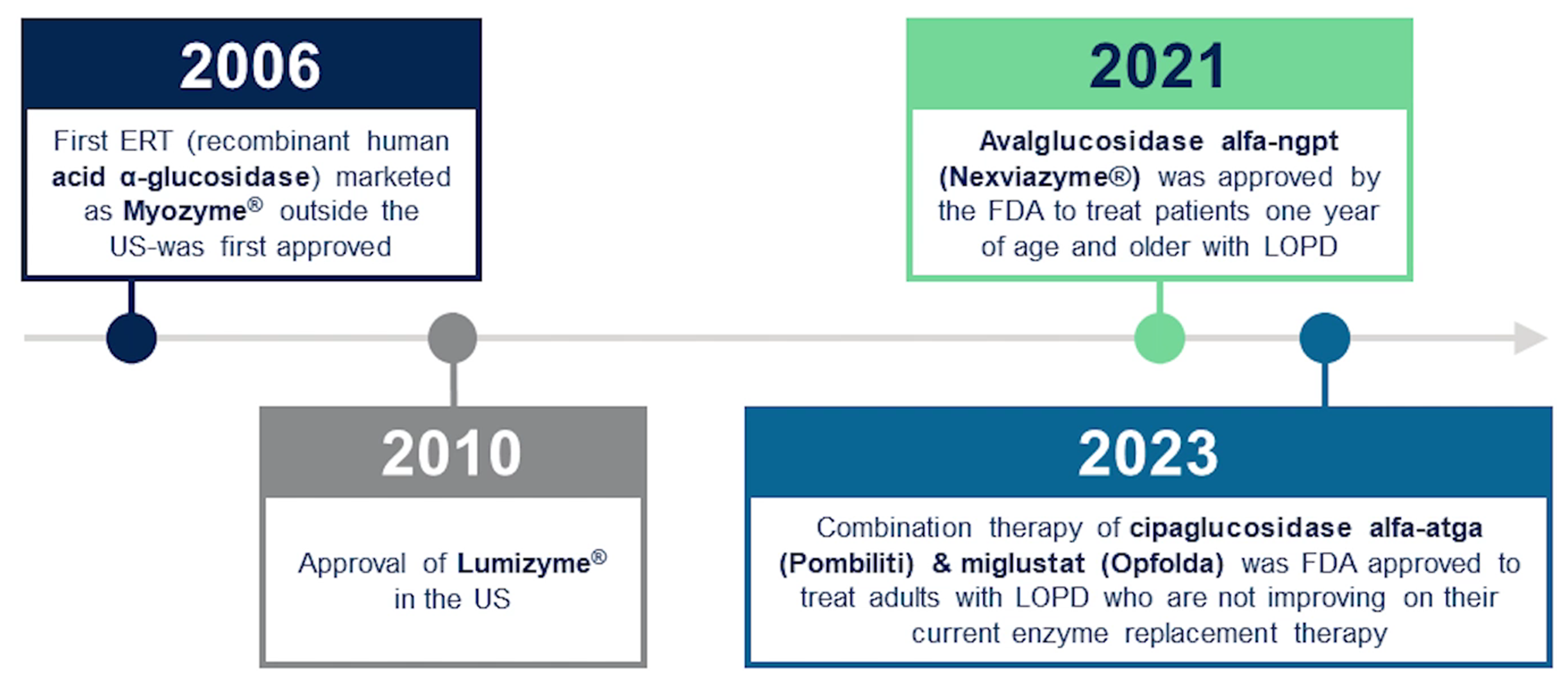
Figure 1. Available enzyme replacement therapies (ERTs) for Pompe disease.
Through continued research, the medical community, industry and the Pompe disease patient community will continue to strive to improve diagnostic accuracy and collaborate in ground-breaking clinical research to expand treatment options and enhance the overall care and quality of life for individuals living with LOPD.
Patient Advocacy in Drug Development
Patient insights in drug development represent a paradigm shift in the healthcare industry, recognizing the invaluable perspectives of individuals living with medical conditions. This collaborative approach fosters a deeper understanding of patient needs and priorities, ultimately informing more patient-centric research and therapeutic interventions. Patients play a pivotal role in shaping the entire drug development process.
At the preclinical stage, conducting landscape analyses of patient advocacy organizations and disease stakeholders to gain insights into disease burden and patient experiences is important. By engaging with patient communities early in the drug development process, companies can foster collaborative relationships and co-create solutions that resonate with patient needs.
During clinical development, it is crucial to integrate patient perspectives into clinical development, protocols and the study design. This includes facilitating educational initiatives, developing advocacy resources and supporting patient recruitment efforts to ensure diverse representation in clinical trials.
Moreover, the webinar underscores the importance of seamless support services and ongoing education during the in-market stage. Sharing patient experiences is also important for gathering real-world insights.
Operationalizing Patient-Centric Rare Disease Studies
Implementing patient-centric studies involves translating patient insights and preferences into actionable strategies to enhance the clinical trial experience. Kristin Black, Clinical Trial Manager at Medpace, shares her expertise on the thorough planning needed to operationalize patient-centricity in rare disease research.
“To ensure success in rare disease research, patient-first planning, or a patient-centric approach, should be top of mind from the start of the study, during study preparation, all the way through the end of the study.”
— Kristin Black
Patient input is vital in shaping study procedures and eligibility criteria to ensure they align with patient needs while maintaining scientific rigor. By collaborating with key stakeholders —including patients and advocacy groups — researchers can tailor study protocols to accommodate the unique challenges faced by individuals living with rare diseases.
“At Medpace, we have a medical team that includes medical monitors and advanced clinical practitioners. They lead indication training with the entire study team. This ensures that all functional areas are aware of the key characteristics of the disease,” explains Black.
“Considerations are made throughout the entire program including factors like case report form (CRF) design or electronic patient-reported outcome (ePRO) assessments.”
Black provides examples of patient-centric approaches (Figure 2).
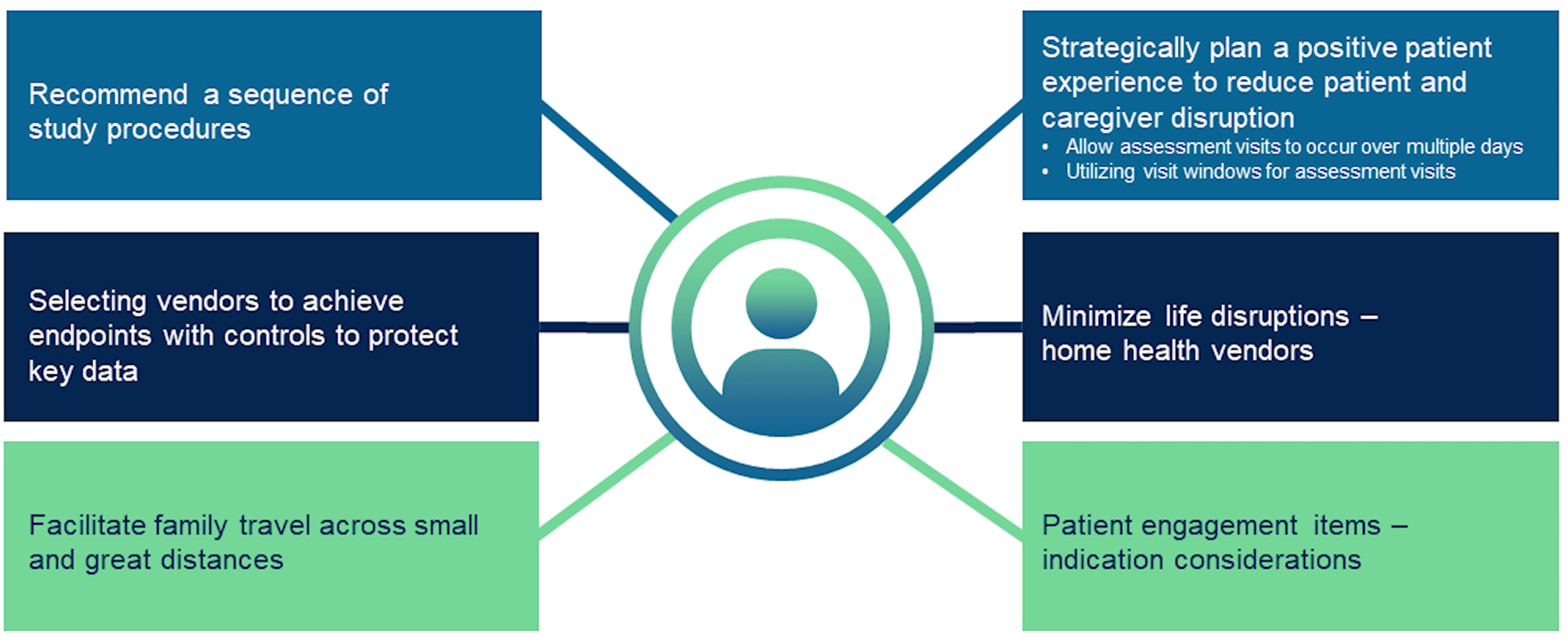
Figure 2. Examples of a rare disease patient-centered approach where the goal is to minimize patient burden.
Black highlights one notable example: a patient-centric approach guided the sequencing of study procedures. Sites were given a recommended order of assessments, with flexibility for adapting to institutional setups and departmental locations. Researchers aimed to optimize data collection and improve the patient experience by prioritizing primary endpoint assessments and including rest periods between procedures.
Furthermore, Black emphasizes the importance of effectively working with vendors to support study operations. This collaboration addresses disease-specific considerations, including implementing contingency plans for challenging assessments. Leveraging vendor partnerships streamlines data collection, improves data quality and accelerates study timelines.
Another critical aspect of operationalizing patient-centric studies is implementing supportive policies and services to alleviate patient burden.
“This includes using our patient concierge service for travel and reimbursement, providing home and/or telehealth options when allowed, using technology, such as the Medpace ePro TrialPACE Patient App, to track and remind patients of visit procedures,” explains Black.
In addition, adapting study procedures to accommodate patient needs is important, particularly in the context of unexpected, uncontrollable circumstances, for example the COVID-19 pandemic. Flexibility in protocol design allowed patients to receive infusions at home and defer assessment visits during illness, ensuring continuity of care and patient safety.
By prioritizing patient well-being and adapting to evolving circumstances, researchers can uphold ethical standards and maintain patient-centricity in study conduct.
Optimizing Patient Engagement for Rare Disease
Patient engagement is paramount in rare disease research, where individuals often face significant challenges accessing information, resources and treatment options.
Miaesha Campbell, Senior Director of Patient Recruitment at Medpace, highlights multifaceted strategies to enhance patient engagement during rare disease clinical trials (Figure 3).
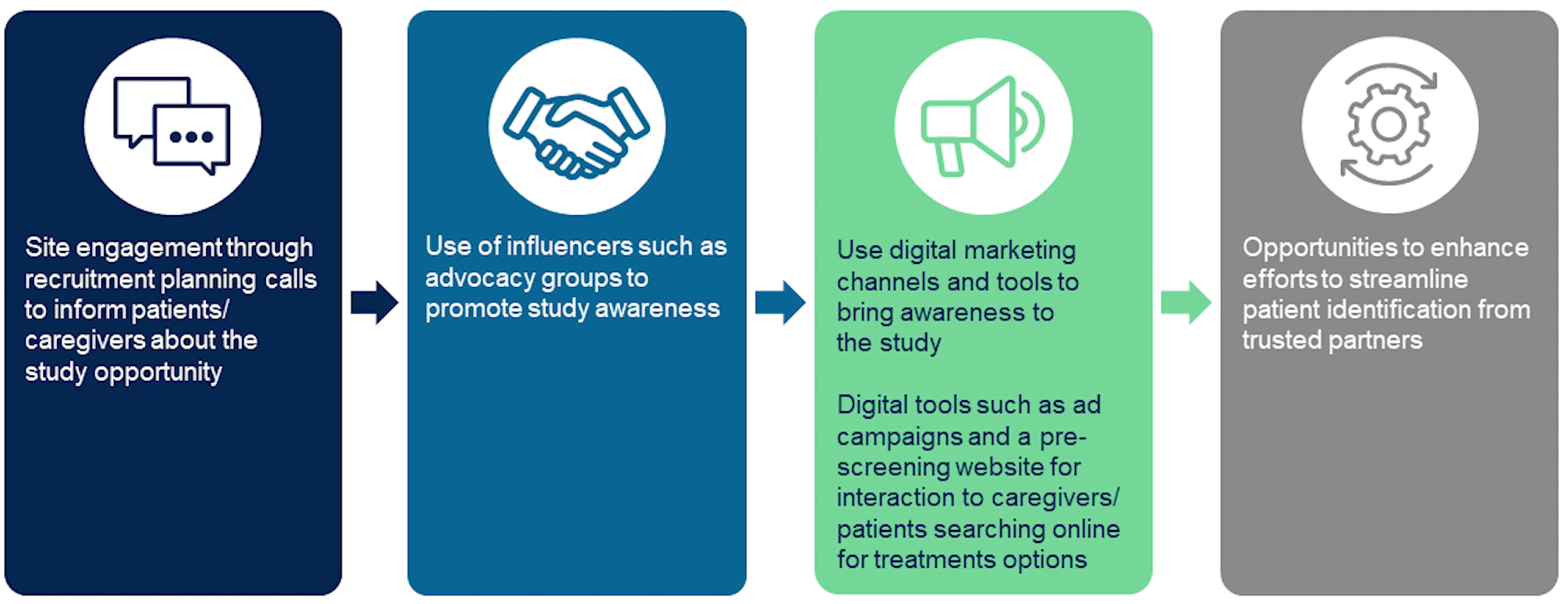
Figure 3. Optimizing engagement for rare disease clinical trials involves using multiple strategies to improve enrollment.
Campbell emphasizes the significance of engaging with sites and advocacy groups to disseminate information about clinical trial opportunities effectively. By collaborating with advocacy groups and leveraging digital marketing tools, researchers can increase study awareness and reach a broader patient population.
One notable approach is using influencers, such as patient advocacy groups, to promote study awareness and facilitate patient education.
“Using all of these channels allows us an opportunity to enhance our efforts to streamline patient identification from these trusted partners and to provide sites with less burden in terms of pre-screening and screening patients.”
— Miaesha Campbell
Interacting with advocacy groups via webinars, newsletters and in-person meetings ensures that both patient and professional advocacy groups are thoroughly informed about the study.
Campbell also emphasizes the importance of leveraging digital marketing tools and online communities to enhance patient engagement. This involves using ad campaigns and pre-screening websites to engage with caregivers/patients who may be searching online for treatment options.
By addressing barriers to participation, researchers can enhance patient retention and foster greater inclusivity in clinical research.
Medpace’s Patient-Centric Approach to Rare Disease Clinical Development and Future Directions
Medpace, headquartered in Cincinnati, Ohio, and founded in 1992, has nearly 6,000 employees and over 30 offices worldwide. Medpace is a full-service Contract Research Organization with a scientifically driven culture that provides therapeutic, operational and regulatory expertise to accelerate global clinical development of safe and effective medicines, especially in rare diseases.
Medpace is committed to engaging patients through out the world to ensure equitable access to clinical research opportunities for new and innovative therapies for all patients affected by rare diseases.
The collaborative efforts of all key stakeholders in rare disease research have led to substantial advancements in treatment options for patients with rare diseases, including LOPD. Through patient-centric study design, inclusive recruitment strategies and ongoing engagement with patient communities, access to innovative therapies has been expanded.
Looking ahead, collaborative efforts hold promise for advancing rare disease research and improving outcomes and quality of life for patients worldwide.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.







Join or login to leave a comment
JOIN LOGIN