The world of neuroscience trials is rapidly evolving, presenting new challenges and opportunities for researchers and clinicians.
The worldwide market for neurology clinical trials stood at a valuation of $5.24 billion in 2022. It’s projected to grow at a compound annual growth rate (CAGR) of 5.6 percent between 2023 and 2030.
This growth is primarily being driven by a rise in neurological diseases like stroke, dementia and peripheral neuropathy, coupled with increased R&D funding for neurological research.
There is currently a heightened emphasis on imaging and liquid biomarkers in neuroscience clinical trials. Additionally, the potential for accelerated approval of therapies via biomarker surrogate endpoints is introducing new complexities into the mix.
“In some areas, say Alzheimer’s disease, we have growing collections of imaging, gene, cerebrospinal fluid (CSF) and blood markers to follow and look at target engagement of our drugs,” says James Vornov, MD, PhD, Vice President of the Medical Department at Medpace.
“But in other areas, and I always think of psychiatric diseases here, they really have yet to yield to this kind of science,” adds Dr. Vornov.
Dr. Vornov recently spoke on a webinar where he discussed the importance of integrating clinical, imaging, lab and other biomarker endpoints in neuroscience trials. He was joined by his colleagues at Medpace, Danielle N. Caudell Stamper, MSN, AGACNP-BC Advanced Clinical Practitioner; Scott K. Holland, PhD, Sr. Director of Scientific Affairs at Medpace Core Labs; and Serena Allen, PhD, Principal Scientist at Medpace Central Labs. Watch the free webinar to hear their expert insights.
Read on to learn more about new key measures relevant to neuroscience trials.
Biomarkers and the Era of Accelerated Approval in Neuroscience
Biomarkers, in essence, serve as indicators of the various biological processes occurring within a patient, including when exposed to a drug. These aren’t direct reflections of a patient’s symptoms severity or their survival. Instead, biomarkers are measures of cellular and molecular parameters that reflect physiological changes underlying disease and drug effects. They might be molecular or histologic markers, or often radiographic measures or physiologic parameters.
In neuroscience trials, biomarkers now play an indispensable role. They offer insights into safety that go beyond what standard medical tests provide. Additionally, they are pivotal in determining target engagement and assisting in dose-finding through observed biological effects. They may also predict efficacy earlier than clinical outcomes can confirm.
Perhaps most crucially, biomarkers are beginning to serve as surrogate endpoints in neuroscience trials, forecasting the potential clinical benefits of a treatment. To follow this, the US Food and Drug Administration (FDA) mandates that sponsors carry out confirmatory studies or Phase IV clinical trials after receiving accelerated approval to validate the anticipated clinical benefit.
For example, the Alzheimer’s drug aducanumab received accelerated approval from the FDA in June 2021 based on the decrease of amyloid beta plaques as a surrogate endpoint. Subsequently, a Phase IV study was initiated to confirm efficacy. However, in this case the study was terminated before completing enrollment because Centers for Medicare and Medicaid Services (CMS) reimbursement approval was granted, leading to insurance reimbursement for prescriptions.
Also, in April 2023, the FDA granted accelerated approval to tofersen for the treatment of adults with amyotrophic lateral sclerosis (ALS) linked to a mutation in the superoxide dismutase 1 (SOD1) gene. This approval was based on the observed reduction of plasma neurofilament light chain (NfL) in patients who received tofersen.
However, biomarkers won’t always predict clinical benefit, as seen with venglustat’s inability to treat GBA1-associated Parkinson’s disease. While the disease’s exact mechanisms are unclear, they might involve glucosylceramide (GL-1) accumulation. Venglustat, a brain-penetrant inhibitor of glucosylceramide synthase, has been shown to reduce GL-1 levels. However, a Phase II study revealed that despite lowering plasma and CSF GL-1 levels compared to placebo, venglustat performed worse on the MDS-UPDRS (Movement Disorder Society – Unified Parkinson’s Disease Rating Scale) compared to placebo.
Therefore, in our continuing efforts to extend the boundaries of medical knowledge and improve patient outcomes, it’s imperative to approach surrogate endpoints with a discerning eye. Recognizing their potential and limitations ensures that clinical decisions remain rooted in a comprehensive understanding of both biology and patient well-being.
Imaging Biomarkers and Endpoints
Imaging biomarkers are fast emerging as surrogate endpoints in many neuroscience trials. The reasons for their rising prominence are manifold.
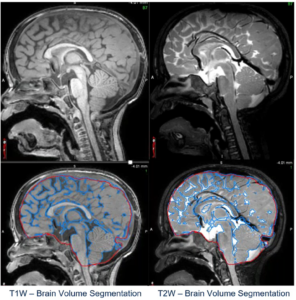
calculation. Figure courtesy of Medpace.
The non-invasive nature of imaging helps to ensure patient comfort and enhance their willingness to be part of trials. The ability to garner insights from imaging in near real-time can significantly accelerate the pace of trials, allowing for timely adjustments and interventions.
Furthermore, the absence of ionizing radiation in magnetic resonance imaging (MRI) makes it a preferred choice for longitudinal studies, capturing changes over time in several brain biomarkers to ascertain the efficacy of a treatment.
Moreover, the adaptability of imaging extends to pediatric applications, a domain where non-intrusive procedures are paramount.
Delving deeper, imaging plays a pivotal role in neuroscience trials in various aspects, including:
- Eligibility: Imaging can serve as a preliminary screening tool to ascertain a participant’s suitability for a trial. It can identify potential safety concerns that might deem a participant ineligible. Furthermore, imaging can help determine if there’s an uptake of a targeted tracer, pointing towards target engagement, for example in radiopharmaceutical trials.
- Safety: Beyond screening for eligibility, imaging provides critical data to ensure patient safety throughout the trial. It aids in optimizing individualized treatment plans by offering insights into brain volume to help inform the selection of the precise dose of the investigational product (IP; Figure 1). Additionally, imaging can help detect any undesirable effects of the IP, for example altered liver size, liver inflammation or potential cardiac issues.
- Efficacy: By comparing with baseline data, clinicians can discern changes in key imaging biomarkers, from the size of brain structures like grey matter, white matter and CSF, to the size of an infarct or the presence of specific proteins like tau or amyloid. Moreover, by comparing these results to control groups, imaging can provide a picture of the treatment’s effectiveness, revealing whether there are improvements in neuroimaging biomarkers.
The non-invasive nature, speed and precision of imaging biomarkers and endpoints position them as invaluable tools, propelling the field toward more comprehensive research outcomes.
Imaging as an Exploratory Efficacy Endpoint in MPS II (Hunter Syndrome)
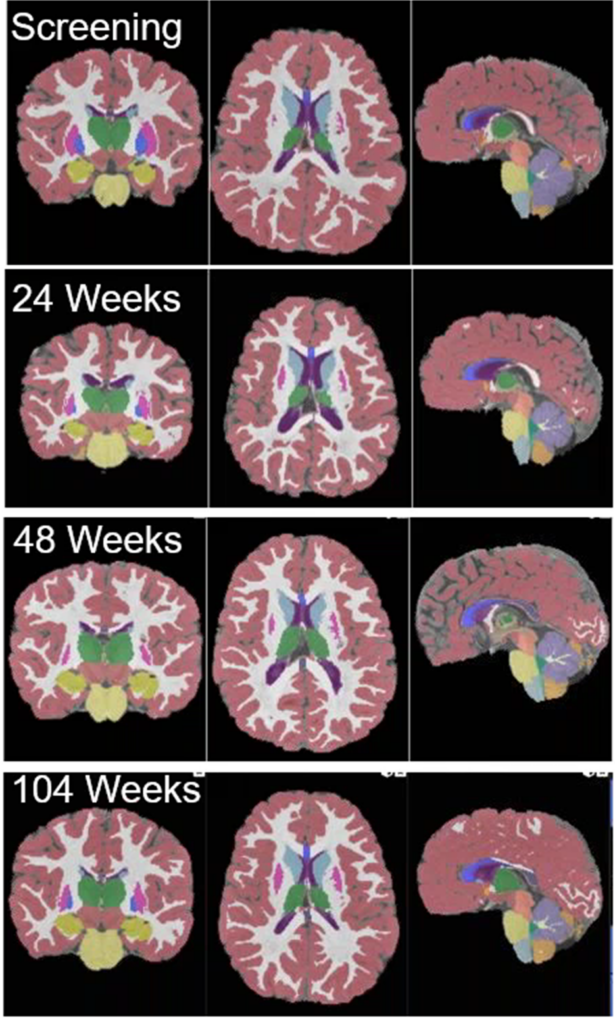
collected during a clinical
trial with MPS II patients.
Figure courtesy of Medpace.
MPS II, also known as Hunter syndrome, is a rare X-linked recessive disease that falls under the category of mucopolysaccharidoses (MPSs).
It’s caused by a deficiency of the enzyme iduronate-2-sulfatase, which breaks down complex molecules called glycosaminoglycans (GAGs). Due to the enzyme deficiency, GAGs accumulate in various body tissues, including the brain, leading to a range of symptoms.
Among the various symptoms and complications related to MPS II, changes in brain volume have also been observed and studied.
According to Dr. Holland, one trial in children employed imaging as an exploratory efficacy endpoint, revealing the capability of the technology in aiding the understanding of this disease and its response to a potential treatment.
During the trial, participants with MPS II received to brain imaging at multiple intervals — namely at the screening, 24 weeks, 48 weeks and 104 weeks (Figure 2). These images, taken from coronal, axial and sagittal perspectives, were segmented and color-coded. The color-coding provided ready visualization to neuroradiologists confirming volumes of pivotal brain regions considered in the assessment of the progression of the disease and response to the treatment.
The segmented and coded regional brain volumes automatically populate an electronic Case Report Form (eCRF) at each time point. These areas included the whole brain, cortical gray matter, cortical white matter, subcortical gray matter, cerebellum and corpus callosum among others. The data is then analyzed with statistical methods to determine the significance of treatment effects.
Dr. Holland says that with the advanced image processing techniques available today, brain volumetric imaging data can undergo unified segmentation, normalization and volumetric analysis. This optimization boosts the sensitivity of the imaging, allowing for more precise detection of changes over time. Such detailed insights, combined with unbiased review processes, can significantly enhance the credibility and reliability of the results, validating the efficacy of innovative treatments in MPS II.
PET Imaging Biomarkers for Alzheimer’s Disease
PET offers quantitative images, providing high sensitivity, especially in the early stages of Alzheimer’s disease. Here are some noteworthy PET imaging techniques for Alzheimer’s disease that Dr. Holland shared during the webinar:
- 18F-FDG PET/CT allows for the measurement of cerebral metabolic rates of glucose. One of its notable characteristics is the ability to detect hypometabolism patterns specific to Alzheimer’s disease. However, it’s worth noting that these patterns can sometimes arise from factors other than Alzheimer’s.
- Amyloid PET enables the visualization of beta-amyloid plaques in the brain, a hallmark of Alzheimer’s disease. It serves to confirm eligibility for certain treatments or clinical trials and is invaluable in distinguishing Alzheimer’s disease from non-amyloid dementias, such as frontotemporal dementia (FTD).
- Tau protein aggregates in the brain can be visualized using Tau PET using specific tracers, including 18FMK-6420, 18F-AV1451 or 18F-T807.
Importantly, FDA-approved products are now available for these PET imaging applications. The approvals have unlocked the potential to use these highly sensitive agents to assess patient eligibility, determine target engagement and evaluate treatment efficacy for Alzheimer’s disease.
PET Imaging for Psychiatry
Recent optimism in the field of neuropsychiatric treatment is well-founded, with the advent of innovative neuroimaging agents that aim to revolutionize our understanding and treatment of complex brain disorders.
Among these is [11C] UCB-J, a promising PET imaging agent that binds to synaptic vesicle glycoprotein 2A (SV2A). SV2A plays a pivotal role in neurotransmitter release and is increasingly being linked to various neuropsychiatric disorders.
Recent studies using [11C] UCB-J have shown an inverse correlation between SV2A density and depressive symptoms in conditions such as major depressive disorder (MDD) and post-traumatic stress disorder (PTSD; Holmes et al., 2019). Furthermore, in patients with chronic schizophrenia, a decrease in SV2A binding has been noted across several cortical regions, indicating its potential as a marker for synaptic density and function.
The quantification of these imaging findings relies on sophisticated analytical methods. The standard uptake value ratio (SUVr) calculation, for instance, uses a pseudo-reference region within the brain — a region that exhibits specific binding, such as the white matter in SV2A PET imaging — to measure the relative concentration of the imaging agent.
The excitement in the neuropsychiatric field is substantial. While there are currently no approved biomarkers for predicting disease outcome or treatment success in psychiatry, the potential of agents like [11C] UCB-J provide hope. These agents not only offer insights into the disease state but also pave the way for monitoring responses to treatment over time.
Biofluid Biomarkers in Neuroscience Trials
The landscape of neuroscience clinical trials is undergoing a transformation driven by the integration of biofluid biomarkers. These sophisticated markers are elevating the capabilities of central labs far beyond their traditional remit of routine chemistry and serology.
Today’s central labs are equipped to offer specialized support for neurodegenerative and other neuroscience conditions by leveraging sensitive and specific biofluid biomarkers. The emergence of these biomarkers is a direct result of groundbreaking technological and research advancements. A single biomarker now has the remarkable ability to provide multifaceted insights into a patient’s condition.
Biofluid biomarkers serve critical functions within neuroscience trials, some of which include:
- Mechanism of action (MoA) confirmation: They help validate that a treatment is correctly influencing the targeted molecular pathways.
- Diagnostic refinement: Biomarkers improve the diagnostic process for neuroscience diseases, enabling early and more precise identification.
- Monitoring treatment effects: They provide a means to evaluate the impact of treatments in real-time.
- Disease progression tracking: With these biomarkers, researchers can observe and measure disease progression repeatedly and accurately throughout the duration of a trial.
- Surrogate endpoint utility: Biomarkers can reveal positive effects of treatments from downstream events, like neurodegeneration.
Moreover, combining biofluid analytes with molecular pathology (genetic biomarkers) allows for the classification and differentiation of neurodegenerative diseases. This is crucial not only for understanding these conditions but also for developing targeted therapeutic strategies.
The Transformative Impact of Blood-Based Assays on Neuroscience Clinical Trials
The advent of ultrasensitive technologies has revolutionized the diagnosis and monitoring of neuroscience disorders, particularly with the introduction of blood-based assays. These assays offer a less invasive alternative to traditional methods, thereby reducing patient burden and potentially increasing the feasibility and frequency of longitudinal monitoring.
Peripheral blood collection, when compared to procedures like lumbar punctures, significantly eases the patient experience. It enables frequent sampling for early and specific neuroscience biomarkers, which can be pivotal in differentially diagnosing diseases at the onset of symptoms, primarily cognitive decline.
During the webinar, Dr. Allen discussed recent advancements that have seen blood-based assays such as blood p-tau181 and p-tau217 gain prominence for their ability to predict tau and amyloid beta-associated pathologies. Notably, Karikari et al. 2020, demonstrated the predictive power of blood p-tau181 for tau and amyloid beta pathologies. Blood p-tau217 has shown effectiveness in distinguishing Alzheimer’s disease from non-Alzheimer’s diseases and has been noted for its comparable accuracy to CSF p-tau and tau PET imaging (Palmqvist et al., 2020).
Furthermore, blood NfL has shown its versatility as a biomarker. Blood NfL has been associated with treatment response in multiple sclerosis, with reductions of blood NfL linked to the clinical effectiveness of drugs (Delcoigne et al., 2020). Blood NfL was identified as a predictive biomarker for the increased risk of multiple sclerosis clinical progression (Bar-Or et al., 2023). For ALS, serum NfL levels have been distinguished for their sensitivity and specificity in separating ALS patients from healthy controls, with ALS patients presenting levels up to 20 times higher, suggesting its potential as a progression marker (Gaiottino et al., 2013).
The utility of blood p-taus and NfL is now extending beyond diagnostics; they are being increasingly incorporated into clinical trial protocols as screening assays for study inclusion, efficacy endpoints and markers to confirm drug target engagement. This shift not only enhances the precision of clinical trials but also opens up new avenues for patient-centric treatment approaches.
Exploring Genetic Biomarkers in Neuroscience Disorders
Genetic biomarkers stand at the forefront of precision medicine for neuroscience disorders, offering insights into the specific gene mutations or variants linked to these conditions. These biomarkers are influential in pinpointing deleterious mutations and allele isoforms that contribute to the phenotypic expression of various neuroscience disorders.
To accurately identify these genetic factors, genotyping techniques such as Sanger sequencing, next-generation sequencing (NGS) and copy number variant analysis are used. These sophisticated methods also serve as a crucial step in screening participants for study inclusion in neuroscience clinical trials.
The table below, shared by Dr. Allen during the webinar, outlines some key genetic markers associated with prevalent neuroscience disorders such as Alzheimer’s disease, Parkinson’s disease and ALS.
Table 1. Genetic markers associated with Alzheimer’s disease (AD), Parkinson’s disease (PD) and ALS.
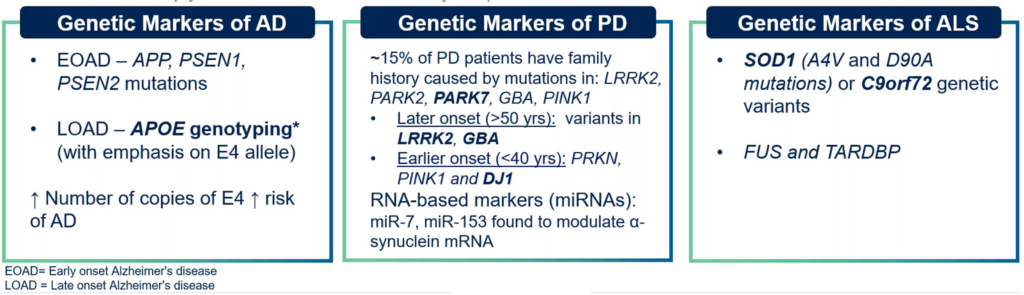
Understanding these markers is vital for the development of targeted therapies and for advancing our grasp of the underlying mechanisms of these complex diseases.
The Integration of Biomarker Endpoints
Modern neuroscience clinical trials rely on the seamless integration of data across clinical observations, imaging, lab results and biomarkers. Reference labs now process diverse liquid biomarker samples, while core labs manage imaging and cardiac safety data and CROs ensure overall data integrity and patient safety.
With the increasing complexity of trials, real-time, integrated data access is essential. Yet, with the power of biomarker data to potentially unblind studies, controlled access remains paramount.
The move from post-trial data collation to real-time analysis has revolutionized trial management, allowing for instant insights into trial dynamics and patient safety. This integrated data approach not only enhances trial efficiency and accuracy, but also ensures patient-centric care.
CRO Strategies for Neuroscience Trials
The success of neuroscience clinical trials is significantly enhanced by the integrated services provided by core labs like Medpace’s core lab services (Figure 3). These labs bring essential imaging expertise to the table, guiding the optimization of imaging protocols and ensuring the sophisticated analysis of data necessary for endpoint determination.
Core labs also ensure the integrity of multi-site trials by qualifying sites, confirming that each one has the appropriate, well-maintained equipment and the capability for precise quantitative assessments. In addition, they handle the critical task of training site personnel in the secure, de-identified upload of data. This is crucial for maintaining patient privacy in accordance with global regulations.
The integrated systems used, such as Medpace ClinTrak®, are designed to protect patient information by removing identifiable data at the source, which is essential for regulatory compliance. The final, and perhaps most vital, phase in the trial process is the expert central review. Performed by board-certified specialists, this review solidifies the reliability of the imaging data as endpoints for the clinical trial.

Figure 3. Achieving success in neuroscience clinical trials with Medpace’s integrated core lab services.
Core lab services are integral to the efficacy of neuroscience clinical trials, ensuring high-quality data collection and regulatory compliance. This ultimately allows for the generation of trustworthy trial results.
Furthermore, the complexity of neuroscience studies necessitates the support of a full-service global central laboratory. Medpace’s full-service global central laboratory brings numerous advantages and considerations critical to the success of neuroscience trials (Figure 4).
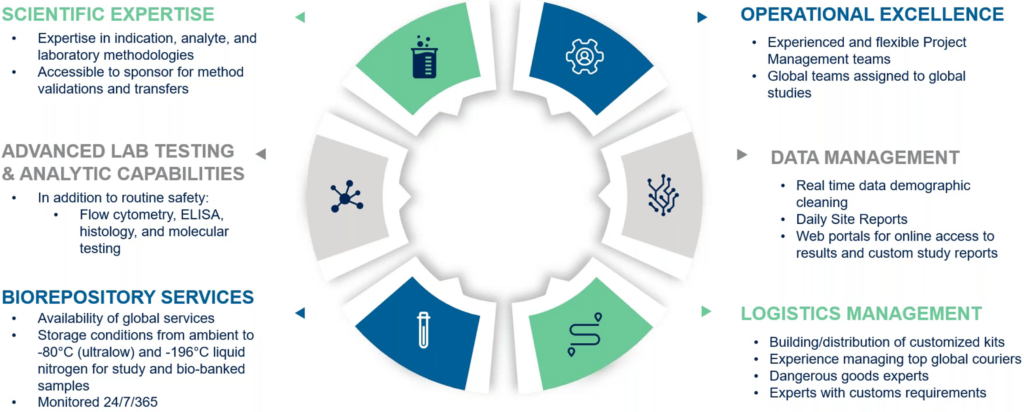
Figure 4. Considerations for neuroscience study support with Medpace’s full-service global central laboratory.
In essence, a full-service CRO is an indispensable ally in advancing neuroscience clinical research. As the field moves forward, implementing integrated core lab services, including global central labs that provide end-to-end support, will remain essential.
With this integrated approach, neuroscience clinical trials are well-positioned to navigate the complexities of research and bring forth groundbreaking therapies.
To gain more insights into how to successfully conduct neuroscience clinical trials, including some operational and logistical case studies from a CRO perspective, watch Medpace’s free on-demand webinar.
References
Bar-Or, A., Thanei, G.-A., Harp, C., Bernasconi, C., Bonati, U., Cross, A. H., Fischer, S., Gaetano, L., Hauser, S. L., Hendricks, R., Kappos, L., Kuhle, J., Leppert, D., Model, F., Sauter, A., Koendgen, H., Jia, X., Herman, A. E. (2023). Blood neurofilament light levels predict non-relapsing progression following anti-cd20 therapy in relapsing and primary progressive multiple sclerosis: Findings from the ocrelizumab randomised, double-blind phase 3 clinical trials. eBioMedicine, 93, 104662. https://doi.org/10.1016/j.ebiom.2023.104662
Delcoigne, B., Manouchehrinia, A., Barro, C., Benkert, P., Michalak, Z., Kappos, L., Leppert, D., Tsai, J. A., Plavina, T., Kieseier, B. C., Lycke, J., Alfredsson, L., Kockum, I., Kuhle, J., Olsson, T., Piehl, F. (2020). Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology, 94(11). https://doi.org/10.1212/wnl.0000000000009097
Gaiottino, J., Norgren, N., Dobson, R., Topping, J., Nissim, A., Malaspina, A., Bestwick, J. P., Monsch, A. U., Regeniter, A., Lindberg, R. L., Kappos, L., Leppert, D., Petzold, A., Giovannoni, G., Kuhle, J. (2013). Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE, 8(9). https://doi.org/10.1371/journal.pone.0075091
Holmes, S. E., Scheinost, D., Finnema, S. J., Naganawa, M., Davis, M. T., DellaGioia, N., Nabulsi, N., Matuskey, D., Angarita, G. A., Pietrzak, R. H., Duman, R. S., Sanacora, G., Krystal, J. H., Carson, R. E., Esterlis, I. (2019). Lower synaptic density is associated with depression severity and network alterations. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-09562-7
Karikari, T. K., Pascoal, T. A., Ashton, N. J., Janelidze, S., Benedet, A. L., Rodriguez, J. L., Chamoun, M., Savard, M., Kang, M. S., Therriault, J., Schöll, M., Massarweh, G., Soucy, J.-P., Höglund, K., Brinkmalm, G., Mattsson, N., Palmqvist, S., Gauthier, S., Stomrud, E., … Blennow, K. (2020). Blood phosphorylated tau 181 as a biomarker for alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. The Lancet Neurology, 19(5), 422–433. https://doi.org/10.1016/s1474-4422(20)30071-5
Palmqvist, S., Janelidze, S., Quiroz, Y. T., Zetterberg, H., Lopera, F., Stomrud, E., Su, Y., Chen, Y., Serrano, G. E., Leuzy, A., Mattsson-Carlgren, N., Strandberg, O., Smith, R., Villegas, A., Sepulveda-Falla, D., Chai, X., Proctor, N. K., Beach, T. G., Blennow, K., … Hansson, O. (2020). Discriminative accuracy of Plasma Phospho-Tau217 for alzheimer disease vs other neurodegenerative disorders. JAMA, 324(8), 772. https://doi.org/10.1001/jama.2020.12134
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.




Join or login to leave a comment
JOIN LOGIN