A daily dose of ibuprofen could prevent individuals from developing Alzheimer’s disease, according to research conducted by Canadian husband and wife neuroscientists, Dr. Patrick McGeer and Dr. Edith McGeer. Their finding that nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen have the potential to be a preventive treatment for the neurodegenerative disease were reported in a recent article published in the Journal of Alzheimer’s Disease.
It’s estimated that over five million individuals in the US suffer from Alzheimer’s Disease, according to the Alzheimer’s Association. Alzheimer’s disease was responsible for an estimated $259 billion burden on the US healthcare system, with this number projected to skyrocket to $1.1 trillion by 2050 as the North American population continues to age.
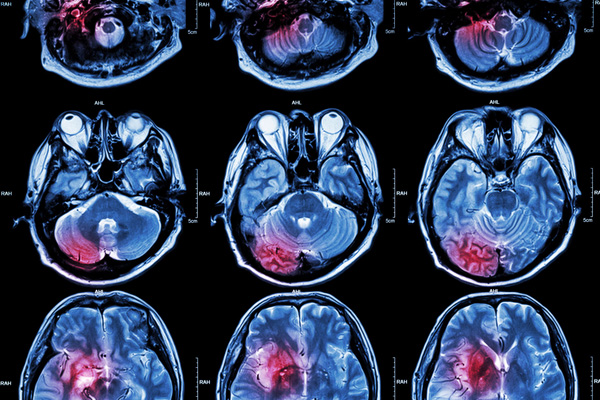
While the causative agent behind the development of Alzheimer’s disease has so far been elusive, researchers have long known that the accumulation of beta-amyloid protein (Abeta 42) is associated with cognitive decline. Since the formation of beta-amyloid plaques in the brain has been linked with increased neuroinflammation, the ideas put forth in the current study have largely relied on the results of NSAID use in those at risk of developing Alzheimer’s disease in previous epidemiological studies.
The McGeers and their colleagues suggest that a simple diagnostic test to measure levels of Abeta 42 in a patient’s saliva could help predict their future risk of developing Alzheimer’s disease, allowing them to take preventive action sooner. The ELISA-based method was developed at the McGeer lab at the University of British Columbia.
“What we’ve learned through our research is that people who are at risk of developing Alzheimer’s exhibit the same elevated Abeta 42 levels as people who already have it; moreover, they exhibit those elevated levels throughout their lifetime so, theoretically, they could get tested anytime,” said Dr. Patrick McGeer, who is also President and CEO of spinoff company Aurin Biotech. “Knowing that the prevalence of clinical Alzheimer’s disease commences at age 65, we recommend that people get tested ten years before, at age 55, when the onset of Alzheimer’s would typically begin. If they exhibit elevated Abeta 42 levels then, that is the time to begin taking daily ibuprofen to ward off the disease.”
But despite Dr. Patrick McGeer’s optimism surrounding the salivary Abeta 42 diagnostic and the potential of NSAIDs to prevent Alzheimer’s disease, others in the dementia community have been critical of the work. A piece published by the NHS in the UK suggests that claims made about this research are “misleading” considering the small study size and the fact that the researchers never actually tested the ability of ibuprofen, or other NSAIDs, to mitigate the risk of Alzheimer’s.
RELATED WEBINAR: Early Alzheimer’s Disease: Finding the Right Patients for Clinical Trials and Beyond
“While we know that targeting new treatments early is likely to bring the most benefits to people with dementia, this approach must be proven in well-designed clinical trials,” said Dr. Carol Routledge, Director of Research at Alzheimer’s Research UK. “While this speculative review claims that ibuprofen could help prevent Alzheimer’s if given early, it does not contribute any new research evidence to assert this claim.”
What’s more, another recent publication by McGeer’s team titled, “Alzheimer’s Disease Can Be Spared by Nonsteroidal Anti-Inflammatory Drugs,” only points to a 1996 review of several epidemiological studies – as opposed to presenting results from original research – as evidence to support their claims surrounding NSAID consumption and the neurodegenerative disorder.
“Population studies, which gather large amounts of information from medical records from thousands of people, have thrown up an idea that taking ibuprofen and other over-the-counter anti-inflammatories might be linked to a lower risk of dementia,” said Dr. Doug Brown, Chief Policy and Research Officer, Alzheimer’s Society. “But results of clinical trials with these drugs have been disappointing so far. The researchers’ suggestion in this paper that taking a daily anti-inflammatory drug as soon as a positive result for dementia risk is shown by a saliva test is premature, based on the evidence at the moment.”
In addition to the lack of supportive clinical trials data, experts are also concerned that recommending daily NSAID therapy to patients who have been found to be at a higher risk of developing Alzheimer’s based on the results of the experimental saliva test could be premature, and even dangerous.
“Any approach to test anti-inflammatory drugs like ibuprofen earlier in Alzheimer’s must be tested in carefully controlled clinical trials to establish safety and efficacy,” said Routledge. “The long-term use of certain anti-inflammatories, like ibuprofen, can increase your risk of other health problems and there is currently insufficient evidence that they are effective or safe to use to prevent Alzheimer’s disease.”
Still, Dr. Patrick McGeer is convinced that their diagnostic is a “game changer” and that providing patients with a non-prescription option to potentially prevent the onset of Alzheimer’s could eventually eliminate the disease.





Join or login to leave a comment
JOIN LOGIN