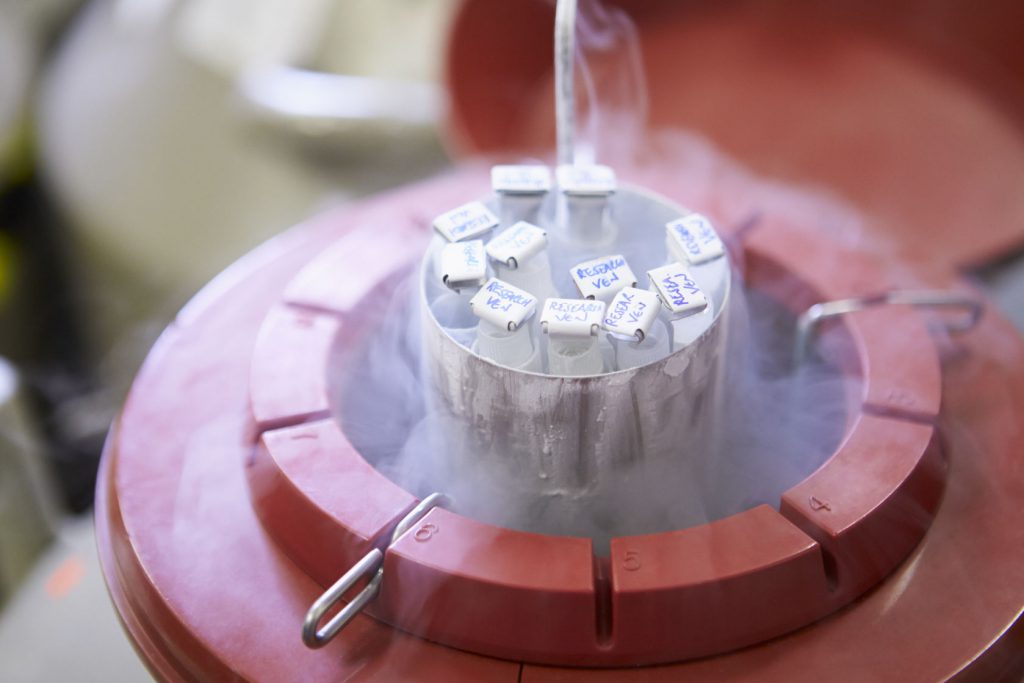
Cell therapy and immunotherapy clinical trials present unique challenges to CROs and clinical sites, including patient safety considerations and complex mechanisms of action. Among the most important components to a successful cell therapy clinical trial is the necessity of controlling the environmental conditions to which the biological product is exposed.
The supply chain for cell therapy clinical trials can be complex, especially if the study involves multiple international sites. To understand how sponsors can reduce risk and position their product well for commercialization, I sat down with Kristen Franklin, Client Services Manager, Cell Therapy and Amy Hendricks, Project Manager, both from Fisher BioServices. Both Kristen and Amy provide strategic supply chain management support for clinical trials and commercialization, and will be speaking in an upcoming webinar on cell therapy development.
What points of contact are involved in the cell therapy workflow, from cell collection to clinical site delivery?
Amy: This is usually customized depending on the client, and whether a CRO is involved. Generally, we do have CROs involved that help to set up or request the shipment, because they’re interacting directly with the clinics. A CRO is responsible for scheduling the supply kit shipments to the clinical sites for collections, and also working with the sites to report when the shipments are collected and distributed to their respective locations. Sometimes the shipments are sent to a CMO who will create the drug, or these shipments can come back to our site or back to the client.
What makes cell therapy more logistically complex, when compared to other drug delivery for clinical trials?
Kristen: The main thing that makes cell therapy more complex from a supply chain perspective, is the requirement to maintain the drug at cryogenic temperatures prior to dosing. In general, the industry standard is that the cell therapy drugs are kept at, or below -120°C. We even have some clients that have a more stringent requirement of below -135° to -150°C.
The challenge there is ensuring that the drug has maintained the appropriate temperature throughout the chain of custody, from manufacturing to short-term storage, to long-term storage, and then finally out to the clinical sites for dosing. We employ a variety of strategies to help maintain that chain of custody and provide supporting documentation. It’s also a regulatory requirement that all temperature information is documented for traceability. The biggest challenge from a logistical standpoint is maintaining the appropriate temperature throughout the chain of custody. This is necessary to ensure that the drug is viable when it gets to the patient.
How does variability in dry shippers affect the integrity of the biological product, and how can you control for that?
Kristen: We control for this variability through qualification testing of all of our shipping equipment. Every shipper that we use to transport drug product is qualified to demonstrate that a particular unit can maintain the appropriate temperature for the duration of the shipment. We also have temperature loggers on each dry shipper so that when the dose does arrive at the site, we have a full temperature report for the life and journey of that dose. The temperature loggers are capable of providing reports that show temperature readings for the entire life of the shipment.
These temperature reports demonstrate that at no point did the container warm up or potentially thaw the material inside, which could impact the potency of the dose. If we do see variability in that testing phase for a shipper, we conduct an investigation and usually rule out that shipper so it’s not used for drug transportation. Only equipment that has passed qualification testing is then used to ship out live drug to patients.
Amy: We also qualify to a specific pack-out per client, and then we do additional testing depending on their needs. For example, we might conduct transit studies to look at worst-case scenarios when shipping to various countries. We also monitor the shippers throughout their lifecycle, including collecting data at time of shipment to ensure that that shipper is able to maintain the qualification specs for that particular shipment.
How do different methods of shipping – such as ground travel or air travel – affect the integrity of the biological product?
Kristen: Generally, the mode of transportation does not have an impact on the integrity of the drug. But in reference to what Amy had said earlier, we do test different methods of shipments when we’re qualifying the shipper. We don’t only test the shipper itself, but also different shipping lanes and shipping methods that it might go through. For example, if we identify that ground travel is a high risk for a particular route or destination, we will proceduralize it to ensure that the preferred method of transport is used to deliver that dose to that site.
How do cell therapy supply chains change as a therapy moves into commercialization?
Amy: The big change is in scalability. The requirements change as the focus shifts towards the ability to scale the supply chain with the different vendors.
Kristen: The supply chain also changes as the scope of the clinical trial changes. We’ve had a lot of clients that we’ve worked with who have started small and gotten bigger. For a Phase I trial, it may just be conducted in the US, however eventually they’ll go global. The challenge we have is taking the strategies we’ve developed with them from a domestic standpoint, and making sure that works internationally.
Amy: One other big consideration that early clinical trials focus on is gathering as much data as possible. As you move further along in the trials, with the idea of going commercial, it becomes more about cost.
What can people expect to learn from your upcoming webinar?
Kristen: One of the big takeaways will be the key points developers should consider when planning a cell therapy clinical trial. We’re going to highlight a lot of challenges and obstacles that we’ve seen, and have been able to overcome.
The earlier you can plan for larger scale operations, standardize your materials, and qualify your shipping lanes, the easier it will be to manage the trial, the cost and the risk down the line. When we’ve worked with clients that really are planning ahead and able to think about those types of things from the beginning, they’re able to implement solutions early on that help them reduce risk and cost throughout the life of the trial.
Amy: We also have a lot of experience with clients in the process of commercialization, who have realized things that they didn’t think of earlier, and we’ve seen the problems caused. Making sure that your standardization is setup from day one, and it’s across all collaborators, is very helpful.
To learn more about cell therapy development strategies and the role of the cold chain, register for Fisher BioServices upcoming webinar.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.







Join or login to leave a comment
JOIN LOGIN