CorNeat Vision’s EverPatch, a synthetic tissue substitute for use in ophthalmic surgery, has received 510(k) clearance from the US Food and Drug Administration (FDA).
EverPatch is the world’s first synthetic, non-degradable tissue-integrating matrix designed for use in eye surgeries.
Ocular surgeries typically involve the use of donor and processed tissue as grafts, which present challenges, namely the risk of disease transmission.
CorNeat’s EverPatch aims to overcome these issues as it is a sterile and non-degradable tissue option.
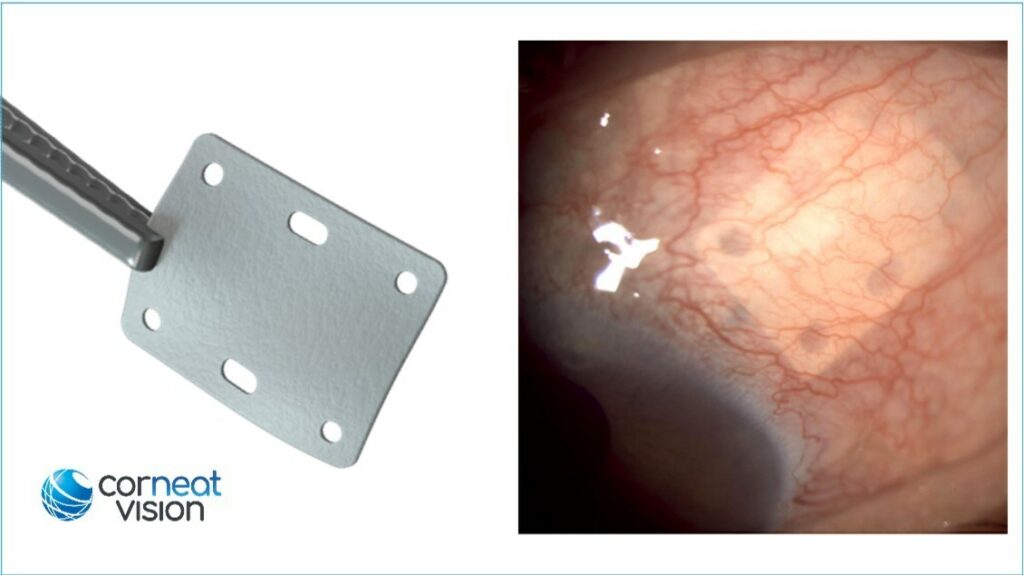
The EverPatch is made of a non-woven, polymer matrix that integrates with surrounding tissue. It is designed to reinforce the sclera and help in the physical reconstruction of the ocular surface.
In a press release announcing the FDA clearance, CorNeat Vision described how the EverPatch is a groundbreaking synthetic tissue substitute that addresses the critical needs of ocular surgeons, providing a sterile and non-degradable solution for patients worldwide.
Related: Miebo Approved as First Dry Eye Disease Treatment to Target Tear Evaporation
CorNeat Vision’s EverPatch is made with the company’s propriety EverMatrix material technology that can integrate with the surrounding tissue.
Dr. Gilad Litvin, CorNeat Vision’s chief medical officer and co-founder explained how the company’s novel ophthalmic patch is “significantly thinner than processed patch tissue, provides better handling as it does not ‘cheesewire’ when sutured and has holes that allow for accurate positioning and anchoring. These holes also facilitate direct conjunctival adhesion to the sclera, thus supporting its bio-integration.”
He also said that the team at CorNeat has received “extremely positive feedback from surgeons and are excited for US surgeons to have this tool available.”
XTALKS WEBINAR: Medical Devices: The Innovation Imperative for Growth Stage Companies
Live and On-Demand: Friday, July 14, 2023, at 1pm EDT (10am PDT)
Register for this free webinar to explore the importance of innovation for growth stage companies and gain valuable insights into strategies for bringing differentiated medical devices to market.
CorNeat Vision CEO and research and development vice president Almog Aley-Raz said, “EverMatrix presents a significant business opportunity as it is the only synthetic non-degradable patch material in ocular surgery.”
He explained that the biocompatible material has potential for wider use in things like soft tissue reinforcement, biomechanical integration of implants with surrounding tissue, engineering membranes and concealing implants and sensors.
The company said the EverPatch will first be launched across some of the top US ophthalmic centers in the third quarter of 2023 followed by nationwide expansion later in the year.











Join or login to leave a comment
JOIN LOGIN