A team from deCODE Genetics, a subsidiary of Amgen, has been working on understanding and analyzing the human genome for the last 20 years. In a new paper published in Science, they unveil their biggest project yet: the most high-resolution map of the human genome.
When the first nearly complete sequence of the human genome was published in 2003 by the Human Genome Project, it was assembled from millions of small fragments that had to be put in the correct order. That ordering was finalized using a genetic map created by deCODE that provided a framework of markers, akin to accurately placed signposts, that told geneticists exactly where they were.
But that genome was only a first reference sequence, consisting of only 6000 markers. The map provided us with only a rough snapshot of our genetic makeup. To gain a richer understanding of our genetic makeup, we must study tens of thousands of whole genomes, not only in full resolution but also over time: as vehicles for transmitting information between generations.
“[This] paper describes the development of a new understanding of how genomes are transported from one generation to the next,” said Dr. Kari Stefansson, CEO of deCODE, in an interview with Xtalks. “In it lies the success of discovering disease genes.”
Release – A Full Resolution Map Of The Human Genome from Íslensk erfðagreining deCODE on Vimeo.
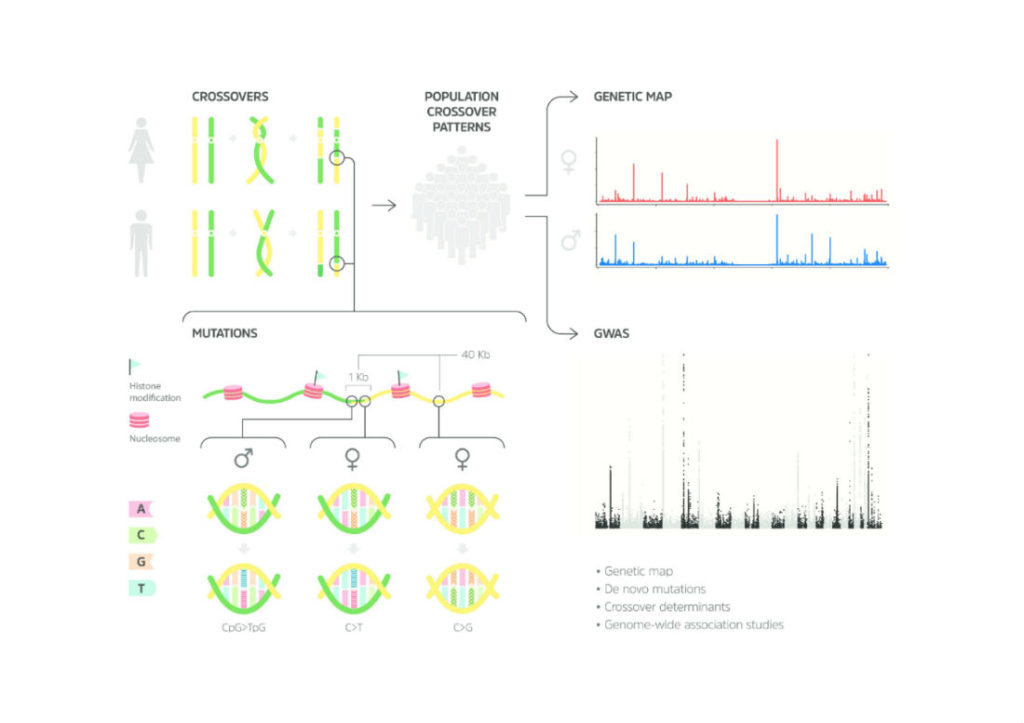
The researchers focused on revealing two key types of changes that occur as genomes are passed from parents to offspring and are important for driving evolution: recombination and de novo mutations.
Recombination, the natural reshuffling of genetic material, occurs during the creation of eggs and sperm. This interchange of chromosomal DNA makes your genome a patchwork of your parents’ DNA, making you similar to but different from your parents, grandparents and siblings, and a completely unique version of our species.
Spontaneous or de novo mutations are those that appear in the creation of eggs and sperm and are not inherited from either parent. De novo mutations lead to many rare diseases, as well as those in which a child might have genetic disorder even when his or her parents do not carry the mutation.
“[True] new diversity is rooted in new mutations,” said Dr. Stefansson. “[However] a proportion of individuals are going to suffer because the mutation happened to hit a gene that is important for survival… de novo mutations are responsible for a large percentage of the disease we see in children.”
Understanding the nature of these mutations can help researchers understand how to treat and prevent disease.
Over the last two decades, the team collected genetic data from over 150,000 Icelanders across multiple generations. They published two genome maps in the past, the one from 2002 and another in 2010, but not with the level of accuracy they were able to achieve today.
“The genetic map shows how bits and pieces of DNA travel together from one generation to the next,” continued Dr. Stefansson. “The theoretical limitation for how accurate this map [can be] is dictated by how close the markers [are] in an individual.”
With their large sample size and access to unique resources, the researchers were able to create a map at 100 times the resolution of their 2002 work. This also means that their data is 100 times more accurate.
The team was able to identify the precise locations of 4.5 million crossover recombinations and over 200,000 de novo mutations. According to Dr. Stefansson, they also found that women contribute far more to recombination while men contribute more to de novo mutations, and it is the latter that comprise a major source of rare, childhood diseases.
But the most surprising finding of all was that these seemingly random mutation events are actually closely connected.
“What surprised us is that these two mechanisms are linked,” said Dr. Stefansson. “What we show in this paper is that in the thousand bases that flank the regions of recombination, the mutation rate is increased by 50-fold.”
In other words, de novo mutations are more than 50 times more likely to occur at a recombination site than anywhere else in the genome. Moreover, the researchers found 35 sequence variants that affect recombination rate and location, suggesting that the genome itself exerts control over the generation of diversity.
While there are no direct implications of the researchers’ findings on gene therapies and genetic editing such as CRISPR, scientists will gain a better understanding of how diseases arise and how genetic material flows across generations. But what about mutations that aren’t passed on?
“Next, we want to use what we learned from the germline genome – what is passed from parent to child – to learn about the nature and distribution of mutations that happen in the somatic genome, the genome contained in the ordinary cells in our bodies that are subject to our environment” said Dr. Stefansson. “[Somatic mutations] are responsible for most cancers… We will use this genetic map to help us in further discovery of disease genes.”
Like a painter creating a fine work of art, mapping out the human genome takes time, patience and a set of specialized tools.
“We have been diligently working on this for a long time,” concluded Dr. Stefansson. “This is just another chapter in a very, very lengthy study.”







Join or login to leave a comment
JOIN LOGIN