There are over 10,000 rare diseases affecting an estimated 300 million people worldwide — where 80% are genetic, 95% lack approved treatments and nearly half begin in childhood.
Global rare disease clinical trials face unique challenges due to small patient populations, complex logistics, diverse regulatory environments and cultural differences.
Reflecting on these challenges, Michelle Petersen, MS, Vice-President of Clinical Trial Management at Medpace, noted, “When it’s rare, it’s personal — every patient counts, and tailored support is key to making a difference.”
In a recent webinar, Petersen and her colleagues at Medpace — James Thomas, BSc (Hons), MA, Senior Director, Regulatory Submissions; Beate Hess, PhD, Sr. Clinical Trial Manager/Sr. Associate Director; and Carly Wolnitzek, Patient Recruitment Manager — shared insights and practical strategies to streamline global rare disease clinical trials involving cross-border patient participation.
Their discussion highlighted the value of proactive planning, robust regulatory intelligence and comprehensive patient support systems. These measures enhance recruitment, retention and overall trial success.
Read on to learn how these innovative approaches are breaking down barriers and reforming global rare disease clinical trials.
The Imperative of Cross-Border Enrollment
Rare disease studies often suffer from limited patient numbers. Expanding enrollment beyond domestic borders is essential for meaningful results.
“Each patient is really precious, so making sure that we can retain them by supporting them and providing them a strong structure that’s thoughtful is critical.”
— Michelle Petersen
International participation not only increases the recruitment pool but also enriches data diversity, leading to more robust and generalizable findings.
Cross-border enrollment captures a wider range of genetic backgrounds, environmental influences, and cultural perspectives. This can improve our understanding of treatment efficacy and safety across populations.
The Challenges of Global Rare Disease Clinical Trials
International rare disease trials are vital for expanding patient recruitment and enriching data diversity, but they come with their own set of challenges.
Some of the challenges of international rare disease trials include:
- Regulatory Variability: Different countries have distinct regulatory frameworks and ethical considerations. Sponsors must navigate varying approval processes, compliance standards and reporting requirements to ensure that trials meet both local and international guidelines.
- Logistical Hurdles: Patient transportation, travel costs and site accessibility can vary dramatically across regions. Effective logistical planning is crucial to coordinate site visits, manage shipment of study materials and support patients who may have to travel long distances.
- Cultural and Language Barriers: Tailoring patient communications and educational materials to diverse cultural contexts is essential. Understanding local customs, languages and healthcare practices can help build trust with participants and improve their overall experience.
- Operational Complexities: Coordinating between multiple stakeholders — including investigators, site staff, regulatory bodies and local patient advocacy groups — requires a well-integrated process. Establishing centralized communication platforms and standard operating procedures can streamline trial operations across different regions.
As Petersen emphasized, “Details are critical to a smooth experience.” Careful planning and collaboration are key to navigating the complexities of global rare disease clinical trials.
Boosting Accessibility Through Specialized Trial Infrastructure
Navigating global rare disease trials demands strong regulatory intelligence, especially as patients cross borders.
As Thomas says, “The accessibility of clinical trials and clinical research is what drives a significant amount of that cross-country movement.”
Global clinical trials for rare diseases have various accessibility needs and considerations (Figure 1).
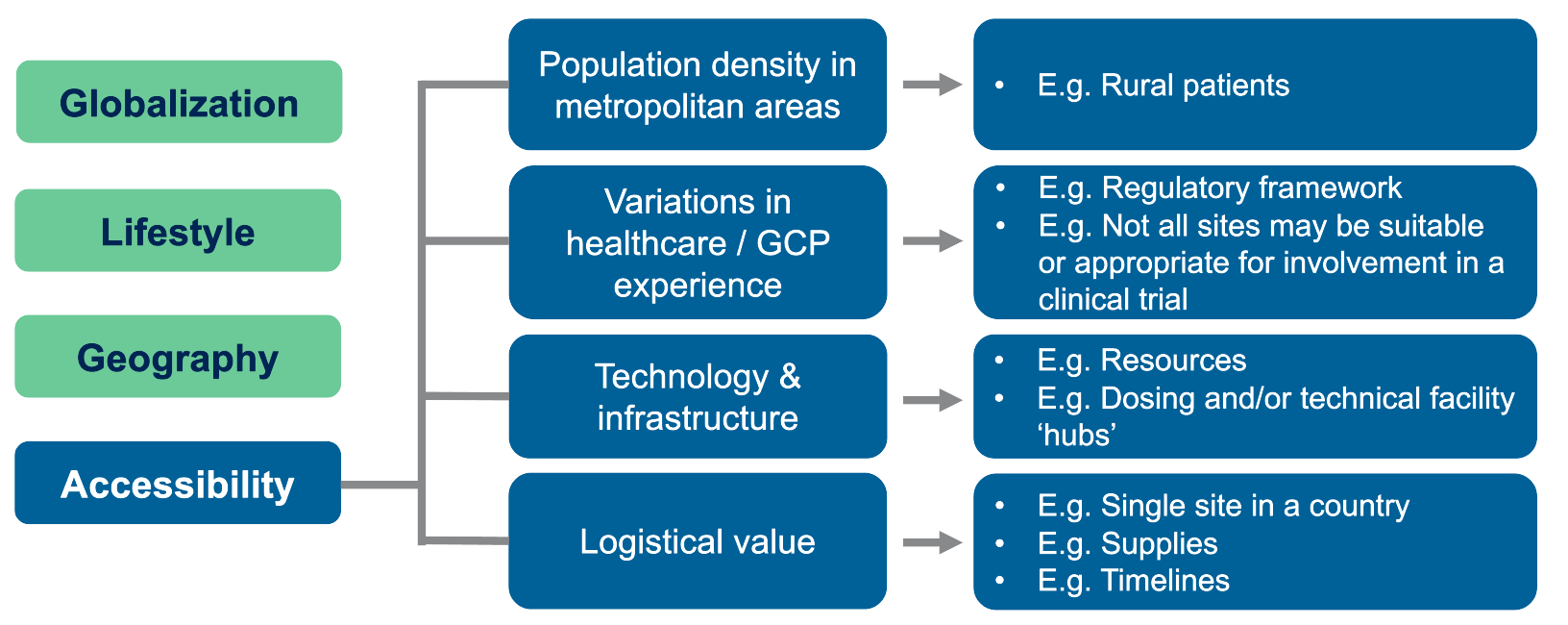
Figure 1. Operational and regulatory need for intra- and inter-country movement.
Metropolitan areas offer many trial sites due to high population density, but rural patients struggle to reach these centers. In addition, differences in healthcare practices and varying GCP experience mean some sites lack the necessary resources to run a trial.
This gap calls for well-planned logistical solutions. Specialized facilities — such as gene therapy dosing centers or advanced MRI sites — can serve as treatment hubs, even if they are far from patients’ homes. In such cases, travel and follow-up care require careful coordination.
As Thomas explains, “For example, we’ve had a number of gene therapy studies where you may be dosing in a specific location, which may not be in the country that patients are generally going to be participating in. So, they may be traveling to be dosed at one time in one location before being followed up for further health care at a different center.”
Researchers must also secure a steady supply of materials and ensure timely distribution. Relying on a single-site model can fail when local logistics restrict access to essential trial resources.
Enhancing Regulatory Intelligence
“Regulations are designed to protect and safeguard. They’re not in place to try and deny healthcare access or access to clinical innovation. So, working with the regulations and having that knowledge is incredibly important.”
— James Thomas
Variation in regulatory frameworks across countries presents a major challenge for global rare disease clinical trials. Each country has its own guidelines, contextual interpretations and multi-layered regulatory requirements.
In this rapidly changing setting, regulatory intelligence becomes indispensable, enabling proactive planning and effective risk mitigation.
Thomas outlines several key regulatory solutions for global rare disease trials (Figure 2):
- Proactive Planning: Conducting up-front risk evaluations, trend analyses and active intelligence gathering allows trial teams to anticipate regulatory changes. Engaging subject matter experts with insights into local regulatory and cultural contexts further refines this approach.
- Dynamic Adaptation: As regulations evolve, clinical trial sponsors must adapt their strategies. Continuous monitoring, detailed impact assessments and using thorough Q&A databases facilitate rapid and coordinated responses to new regulatory requirements.
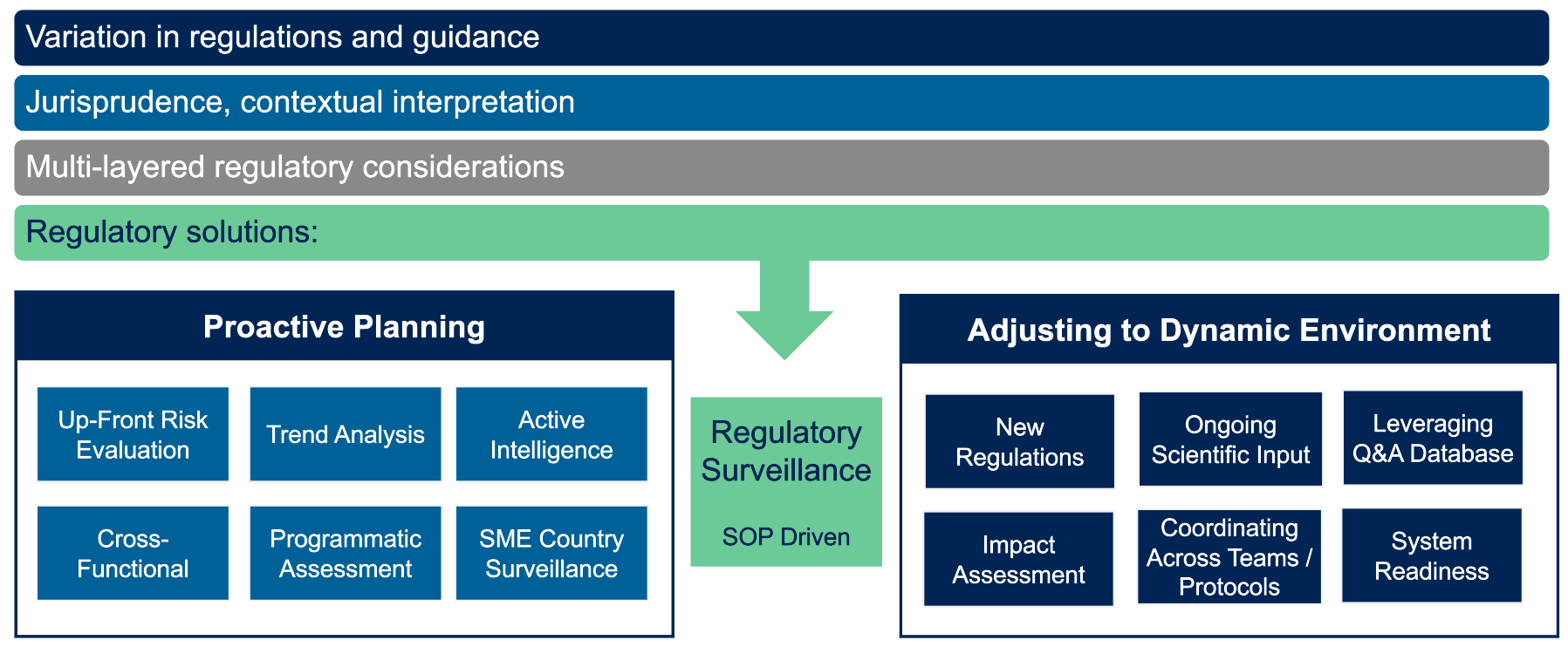
Figure 2: Regulatory environment: Importance of regulatory intelligence.
Thomas further illustrates these concepts with real-world examples. In gene therapy studies, for instance, patients may need to travel to specialized dosing locations outside their home country. Coordinating such logistics requires a careful balance of regulatory and practical considerations.
“Having a strong regulatory knowledge, particularly for technical gene therapy trials where you might have a hub location, allows that expertise to be integrated into feasibility assessments, country and site selection and study design upfront,” explains Thomas.
In practice, some key regulatory considerations for global rare disease trials include:
- Connected Approach: Engage all stakeholders and review bodies — including regulatory authorities, ethics committees and additional review boards — to facilitate successful outcomes.
- Document Development and Knowledge: Ensure compliance by thoroughly reviewing insurance policy terms and conditions, tracking key details and commitments and collaborating directly with brokers.
- Technical “Hubs”: Integrate specialized expertise, particularly in gene therapy, into feasibility assessments, regulatory process understanding, patient logistics and ancillary support.
- Patient Materials: Develop informed consent forms and other patient information with proactive contingency planning to accommodate various scenarios while maintaining financial consistency.
As Thomas emphasizes, the movement of patients across borders is a given; the key lies in ensuring that every aspect of trial design is agile enough to support this reality.
Operational Excellence: Global Perspectives and Strategies
Global rare disease clinical trials present unique challenges that require a strategic approach to site selection, staff training and patient support.
Dr. Hess shares the key operational considerations that can streamline trial conduct and enhance patient retention and data quality.
Site Selection: Balancing Expertise and Accessibility
“Selecting the right sites and training of staff to meet diverse patient needs are critical to overcoming the logistical and cultural complexities of global rare disease studies,” says Dr. Hess. This integrated approach ultimately reduces patient burden while enhancing overall efficiency and success (Figure 3).
Leading sites are typically located in major urban centers where key opinion leaders and disease specialists practice, yet these centers may be less accessible to patients in rural areas. This challenge necessitates a broader geographical approach that accounts for both expertise and accessibility.
Regulatory barriers, along with cultural, religious and ethical influences, can also affect the suitability of a site. Tailoring the trial approach through language/culture-specific questionnaires and culturally adapted consenting processes is essential to address these nuances.

Figure 3. Key considerations for successful site selection.
In addition, patient logistics are critical — factors such as travel time, patient burden and the choice between relocation and travel must be carefully considered. Leveraging modern techniques like remote consenting, self-triggered travel platforms and home healthcare services can help minimize disruptions and allow patients and their families to remain focused on the study.
Training Site Staff for a Diverse Population
Site staff training is critical to managing the intricacies of global rare disease trials. According to Hess, training programs should focus on:
- Cultural, Language and Religious Competence: Equipping staff with knowledge of cultural differences and language barriers helps in creating patient-friendly educational materials and ensuring an empathetic consent process.
- Awareness of Patient Challenges: Staff must be trained to understand the practical challenges patients face, such as managing travel logistics, navigating time zone differences and adjusting to new dietary and social environments.
- Using Support Materials: Providing comprehensive educational materials, quick reference guides and checklists ensures uniformity in trial conduct and improves both patient retention and data quality.
“Effective training bridges the gap between logistical challenges and cultural understanding, ensuring that every team member is prepared for the diverse needs of rare disease trials.”
— Beate Hess
Dr. Hess’ insights reinforce the importance of comprehensive training of site staff that integrates both patient-centric logistical support and cultural competence.
By investing in such tailored training initiatives, sponsors can equip site teams to overcome the unique challenges of rare disease studies.
Enhancing Study Staff Support
Another critical factor is the early and ongoing involvement of study staff. Their role in communicating trial protocols and managing patient expectations cannot be overstated. A robust support system not only improves patient retention but also enhances data quality across the study.
Hess suggests considering these operational strategies:
- Integrated Logistical Platforms: Using modern technologies, such as patient self-triggered platforms for travel and accommodation, can simplify many operational challenges.
- Consistent Communication and Monitoring: Regular updates, quick reference guides and dedicated patient concierge services help manage both patient expectations and logistical hurdles effectively.
Such initiatives ensure that the trial environment remains as stress-free as possible for both patients and staff, leading to better overall outcomes.
Patient and Caregiver Engagement
Patient and caregiver engagement is also an essential operational strategy. Clear, accessible educational materials and robust support mechanisms empower participants to commit fully to the study protocol. Rare disease trials benefit from a proactive approach to involvement, as illustrated in Figure 4.
Providing detailed educational materials that clearly explain study participation, including its risks and benefits, is fundamental. In addition, offering flexible scheduling and support services to address logistical challenges — such as travel and childcare — ensures that patients can participate without undue burden.
Moreover, leveraging patient advocacy and support groups helps tailor communication and resources to the unique needs of the rare disease community.
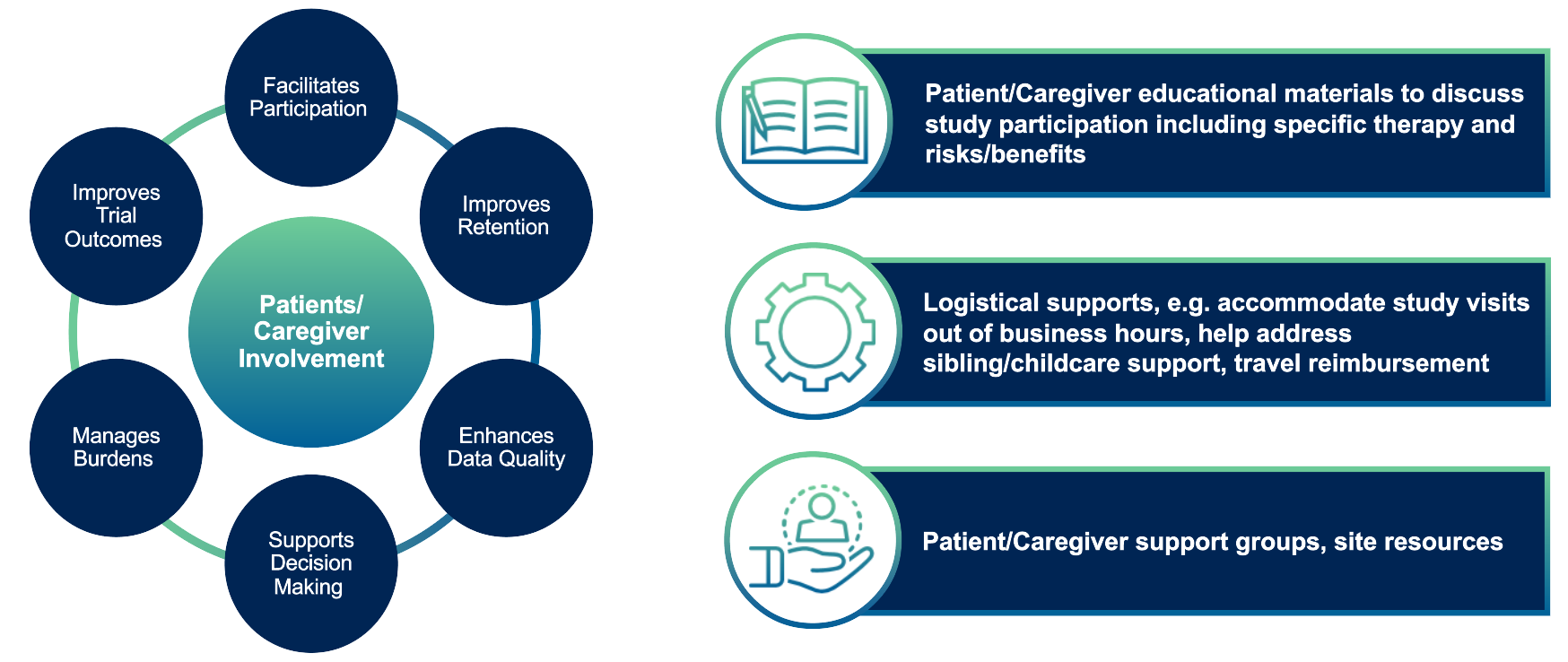
Figure 4. Patient/caregiver support and education.
By keeping patients and caregivers well informed, trials not only improve retention but also enhance the quality and reliability of the collected data.
Patient-Centric Strategies for Cross-Border Clinical Trials
Global rare disease clinical trials face challenges that extend far beyond clinical procedures.
Patients and caregivers confront financial burdens, logistical complexities and emotional strain.
This situation highlights the need for a holistic, patient-centered approach in cross-border enrollment.
Understanding the Patient Perspective
Wolnitzek explains that patients and their families face several challenges. For example, long-term travel, lodging and incidental expenses can escalate quickly. Moreover, extended absences from work and family create scheduling dilemmas.
In addition, coordinating multi-modal transportation and various service providers demands meticulous planning. Managing a health crisis in an unfamiliar country can overwhelm patients emotionally.
By addressing these issues from the patient’s point of view, clinical teams can design strategies that boost enrollment and improve retention.
Establishing a Patient-Centric Plan from the Start
Effective patient support begins during the study and site start-up phase. A comprehensive plan that mitigates financial, logistical and emotional barriers should be built on three key pillars:
- Travel & Payment Support: Implementing a full-service travel solution is essential. Covering everything from flights and ground transportation to lodging, visa assistance and timely reimbursements helps reduce the financial strain on patients and caregivers.
- Support Systems: Establishing a robust network — including clinical site staff, dedicated travel partners, caregiver support and local or online patient groups — helps ensure that patients never feel isolated in their journey.
- Education & Awareness: Providing clear, detailed educational materials about the study journey, reimbursement processes and available support helps reduce anxiety and build trust.
Case Study: Phase I/II Pediatric & Adolescent Trial Example
Dr. Hess shares a practical example of these principles in action from a recent Phase I/II study involving pediatric and adolescent patients (Figure 5).
This study focused on a rare disease with no existing treatment, where the sponsor opted for a single site approach. The advantages of having identical personnel and consistent assessments were balanced by the challenge of long travel distances for some patients.
Initially designed as a one-year study with weekly site visits to manage a high volume of data per patient, the protocol was later extended for several years, maintaining the same intensive weekly schedule.
“It is not about if these patients will travel, it is about when.”
— Carly Wolnitzek
Despite the increased travel demands over time, the robust support arrangements in place ensured that patients and caregivers felt well cared for throughout the study. This inclusive support helped transform what could have been a significant burden into an appreciated and manageable commitment.
Moreover, the strong global connectivity among affected families fostered widespread enthusiasm, ultimately leading to increased participation requests from around the world and additional considerations for travel and relocation.
As Dr. Hess observes, “Affected families are intensely connected worldwide: Families spread study information; families request participation.”
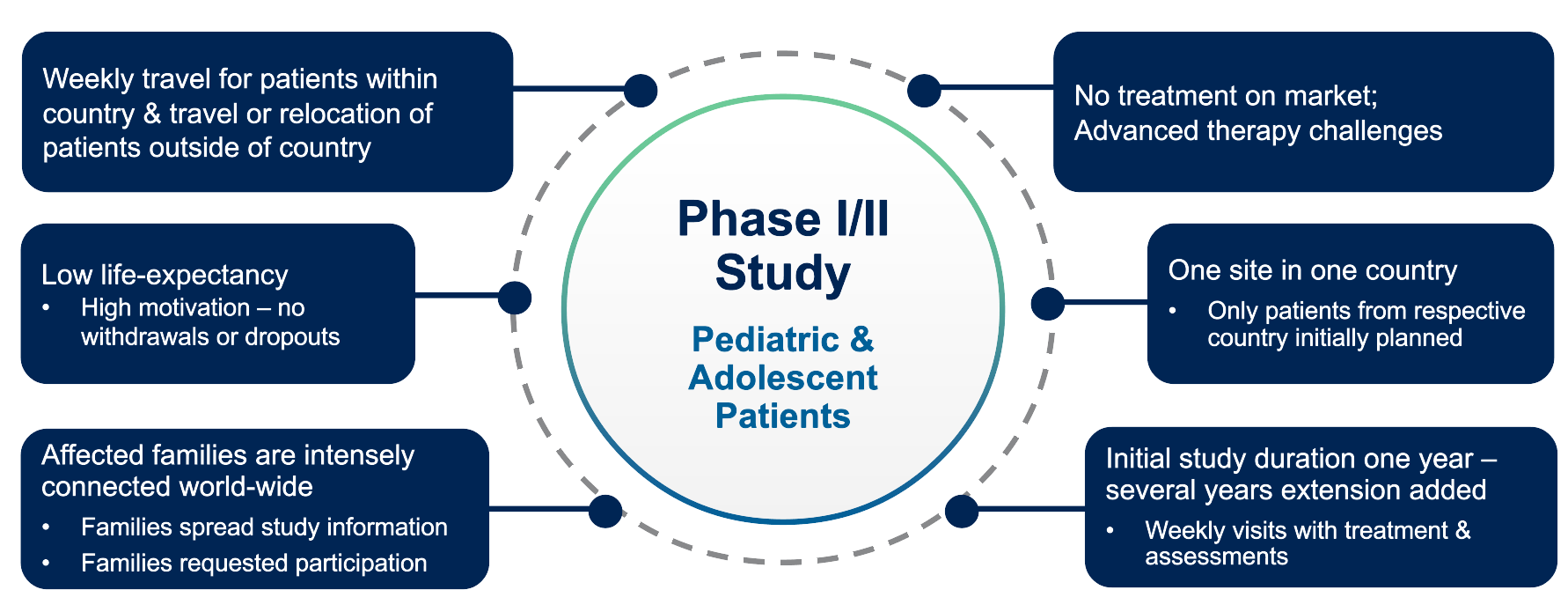
Figure 5. Cross-border participation case example.
The study’s success in patient retention — evidenced by zero dropouts — underscores the critical importance of a well-implemented, patient-centric approach, even in long-term, highly complex clinical trials where the stakes for families are exceptionally high.
As clinical research advances, these regulatory, operational and patient-centric strategies will be crucial for overcoming the unique challenges of global rare disease studies.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN