Mental health problems represent a critical area of unmet need worldwide, affecting an estimated one in eight people globally, according to the World Health Organization (WHO). These issues carry substantial personal and societal costs, impacting daily life and productivity. In fact, mental health conditions are associated with a 10- to 25-year reduction in life expectancy, emphasizing the need for effective and accessible treatments.
Despite advancements in neuroscience and psychiatry, clinical trials for mental health conditions — such as major depressive disorder (MDD), schizophrenia, bipolar disorder and generalized anxiety disorder — face unique challenges. The range in symptom presentation and the variability in treatment response make a successful psychiatry trial particularly complex, requiring an innovative, patient-centered approach.
Reflecting on these complexities, Dr. James Vornov, Vice President of the Medical Department at Medpace, remarked, “It’s more than just people not feeling well or not functioning well. It’s integral to people’s well-being to have a good mood and motivation in daily activities with their families and work life.”
In a recent webinar, Dr. James Vornov and his colleagues at Medpace — Jill Adkins, Sr. Director of Clinical Trial Management; Daniela Rae, MSc, RGN, IP, Advanced Clinical Practitioner; and Miaesha Campbell, Sr. Director of Patient Recruitment — discussed innovative, patient-focused strategies designed to equip psychiatry trials for delivering meaningful, lasting results in mental health treatment.
Read on to learn how these experts at Medpace are addressing the complexities of psychiatry clinical trials with patient-centric approaches that enhance recruitment, retention and inclusivity across diverse patient populations.
Unique Challenges in Psychiatry Clinical Trials
Psychiatry clinical trials face distinct challenges that set them apart from other therapeutic areas. These trials must navigate the complex and subjective nature of mental health symptoms and high placebo response rates, which can significantly impact trial outcomes.
One of the main challenges lies in defining and selecting appropriate trial populations. Unlike other diseases with objective markers — such as blood pressure for cardiovascular trials or imaging results for cancer trials — psychiatric conditions are primarily diagnosed and assessed through patient-reported outcomes (PROs) and clinical observations.
As Dr. Vornov explained, “The population [in psychiatry trials] is really different than most other medically defined diseases … here we’re talking about how people feel, their subjective states.”
This subjectivity introduces variability that complicates both diagnosis and outcome measurement, making it difficult to standardize criteria and ensure consistency across participants.
“Especially as we exited the blockbuster drug period of psychiatric development and trials were failing with huge placebo effects, I think we started realizing that the patients going into trials were not the real-world patients that were getting treated with drugs in the clinic.”
— James Vornov
Moreover, psychiatry trial populations often don’t fully represent the broader, real-world community of patients. For instance, many trials exclude individuals with comorbid conditions. This can result in findings that lack applicability to the broader patient population seen in clinical practice. To bridge this gap, it’s essential that trial designs aim to include diverse and representative participants who better reflect real-world demographics.
Another unique challenge in psychiatry trials is the placebo response, which is notably high in mental health research. Conditions such as depression and anxiety often respond positively to placebo treatments due to the subjective nature of symptom assessment and patients’ expectations of improvement.
This strong placebo effect can mask the true efficacy of the treatment, leading to trial failures. To mitigate this, robust trial designs that include rater training and placebo response education can help improve the chances of detecting a true treatment effect.
Finally, psychiatry clinical trials must also address the variability in treatment responses. Each patient’s experience with mental health conditions is influenced by unique genetic, psychological and environmental factors.
As Dr. Vornov noted, psychiatry trials are increasingly adopting a personalized approach to better meet individual needs: “We’ve realized over the years in these trials that one size doesn’t fit all … very often, our trials have involved patients who have failed everything and are trying a new treatment, where now we understand that genetic, environmental and neural mechanisms may cause a patient to respond better to one catecholamine inhibitor, for example, than another.”
By understanding these distinctions, trials can enhance effectiveness and ultimately lead to more impactful therapies.
Addressing the Complexity of Psychiatric Illness in Clinical Trials
Psychiatric illnesses present unique challenges in clinical trials due to their complexity and the diverse needs of participants (Figure 1).
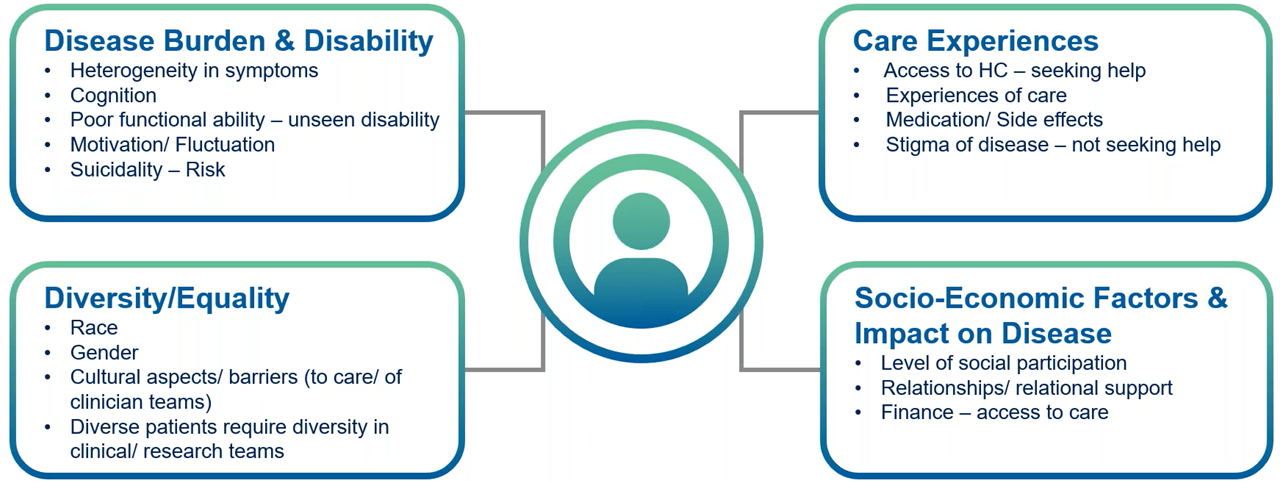
Figure 1. The complexities of psychiatric illness can impact the clinical trial experience.
Daniela Rae explained that the effects of psychiatric illness on cognition is often under-recognized, as individuals commonly experience poor concentration and memory difficulties.
Symptoms like reduced memory, concentration and poor overall executive functioning hinder participants from adhering to medication regimens, following study schedules or providing informed consent. Rae emphasized the need to “empower and enable research sites to accommodate patients rather than patients accommodating study schedules.” Practical adjustments, such as layering consent processes and using decision-making aids, help participants understand and engage with trial requirements throughout the study.
“It is important to empower and enable research sites to accommodate patients rather than patients accommodating study schedules.”
— Daniela Rae
Diversity and inclusivity also play a critical role in psychiatric trials. Factors such as race, gender, cultural norms and socioeconomic barriers often affect access to care and willingness to participate in research.
For instance, cultural stigmas may discourage certain populations, including men and LGBTQ+ individuals, from seeking treatment or joining trials. Rae stressed the importance of increasing diversity within research teams to build trust and better represent underrepresented groups in clinical studies.
Financial constraints create another significant barrier to participation, particularly for patients struggling with the economic impact of mental health conditions. Rae highlighted this challenge: “Loss of ability to work or work at all times … makes participation maybe just too costly.”
Providing practical support such as transportation reimbursements and flexible scheduling not only makes trials more accessible but also addresses the financial realities many participants face. These accommodations are critical for enabling participation from socioeconomically disadvantaged groups, which are often underrepresented in psychiatric research.
Finally, robust safety measures are vital for addressing the risks associated with psychiatric conditions, such as suicidality. Rae shared an example from a recent trial involving participants with major depressive episodes, where the Columbia Suicide Severity Rating Scale (CSSRS) was implemented to monitor safety trends and provide proactive interventions.
“Keeping the participant at the center when thinking about suicidality, suicidal ideation or exacerbation of disease presentation — a good starting point is to consider what is normal for a population and what is not in line with what we would expect to see,” Rae explained. By tailoring safety protocols to meet the specific needs of psychiatric patients, trial teams can ensure both ethical conduct and reliable outcomes.
Patient-Centric Trial Design and Operational Strategies
In psychiatry clinical trials, patient-centric design is vital to address the unique needs of participants and improve recruitment, retention and trial success. By prioritizing patient comfort, understanding, and engagement trials can enhance data quality and minimize dropout rates.
A core component of patient-centric design is comprehensive education and communication. Patients in psychiatric trials often encounter unfamiliar concepts like placebo-controlled designs, PROs and clinician assessments. Providing clear explanations about data privacy, treatment protocols and trial expectations builds trust and fosters engagement.
As Adkins pointed out, “We need to be thinking about how best to support our patients in providing honest, accurate responses to questionnaires and assessments. Otherwise, the data may be inaccurate and really isn’t going to be useful.”
To reduce patient burden, trials increasingly use decentralized clinical trial (DCT) technologies, such as telehealth visits, eConsent and electronic PROs (ePROs). These tools allow patients to participate from home, making trials more accessible to those with mobility or transportation challenges. For example, patients can complete assessments via ePROs, reducing clinic visits and broadening the participant pool. Flexible and remote options also improve accessibility for patients balancing full-time jobs or family obligations.
“We really need to strike a balance between making sure we’re collecting the desired information to support our endpoints, but also avoiding having overly burdensome study visits.”
— Jill Adkins
Streamlining trial protocols further alleviates the burden by reducing visit length and frequency and focusing on essential assessments. Shorter questionnaires and fewer redundant evaluations help maintain patient interest while ensuring data quality.
Another key strategy in patient-centric trial design is effective placebo response management. Psychiatry trials often struggle with high placebo responses, as patients may report symptom improvement based on expectations rather than the treatment itself. Trial teams address this challenge by providing rigorous rater training and educating both patients and clinicians about the role of placebos. This training helps manage expectations, reduce biases in patient responses and ensure consistent, reliable assessments across study sites.
Active participant support is equally critical. Trial staff help patients adhere to protocols through reminders, check-ins and clear instructions. Digital tools and wearable devices further assist with adherence, capturing critical data without increasing burden. Practical support, such as transportation reimbursements, flexible scheduling and meal cost assistance, also makes participation more feasible.
As Campbell emphasized, “Providing services that remove the barriers and burdens of participation creates a study-friendly ecosystem that keeps patients engaged and motivated to continue.”
By integrating educational resources, flexible protocols and tailored support systems, psychiatry trials can enhance patient experiences, improve retention and generate high-quality data.
Empowering Sites and Supporting Patient Engagement
Trial teams can empower sites by providing training, resources, and support systems that not only improve patient engagement and retention but also strengthen endpoint protection and ensure high-quality data collection. This collaborative approach aligns site staff, sponsors and patients to achieve shared goals, fostering a trial environment where accurate and reliable outcomes are prioritized (Figure 2).
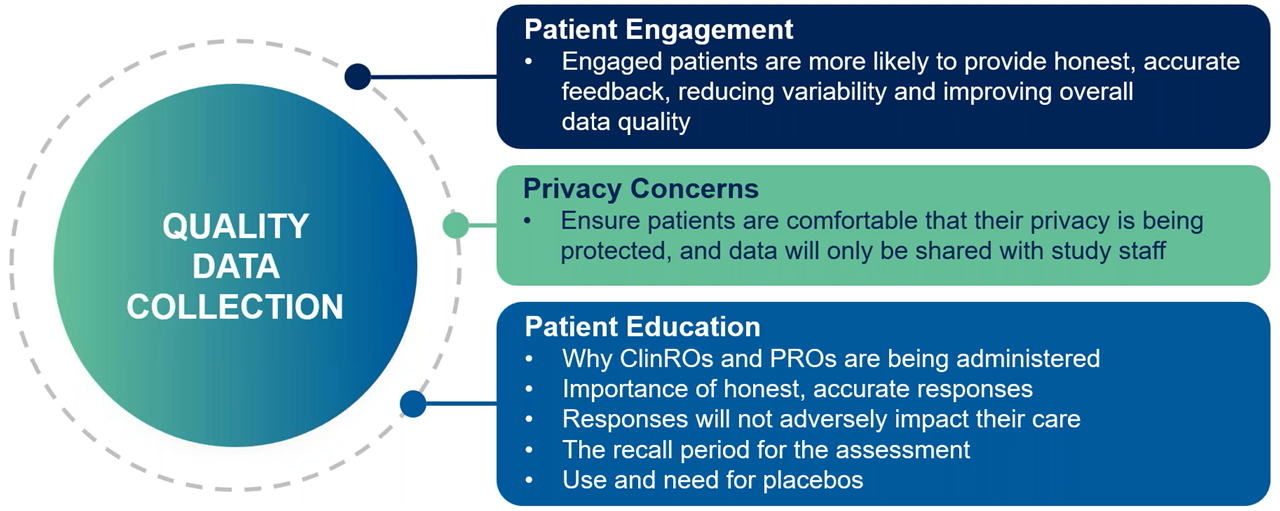
Figure 2. Endpoint protection in psychiatric clinical trials: Improving data quality through patient engagement, education and training.
Robust training and standardized resources equip sites to manage the complexities of psychiatric trials. Consistent rater training minimizes variability and improves the reliability of subjective outcomes.
Adkins emphasized the importance of site support, saying, “Quality study manuals, tools and support are essential to equip sites with the knowledge and resources they need to recruit and retain the right patients.” Detailed study manuals and pre-screening checklists streamline recruitment and patient management.
Ongoing engagement with site staff is critical. Regular updates, feedback sessions and open communication help address challenges proactively and keep sites aligned with trial objectives. Empowered sites can focus on recruiting suitable participants who align with study goals rather than simply filling quotas. By fostering intentional recruitment practices, sites enroll engaged participants who are more likely to complete the trial (Figure 3).
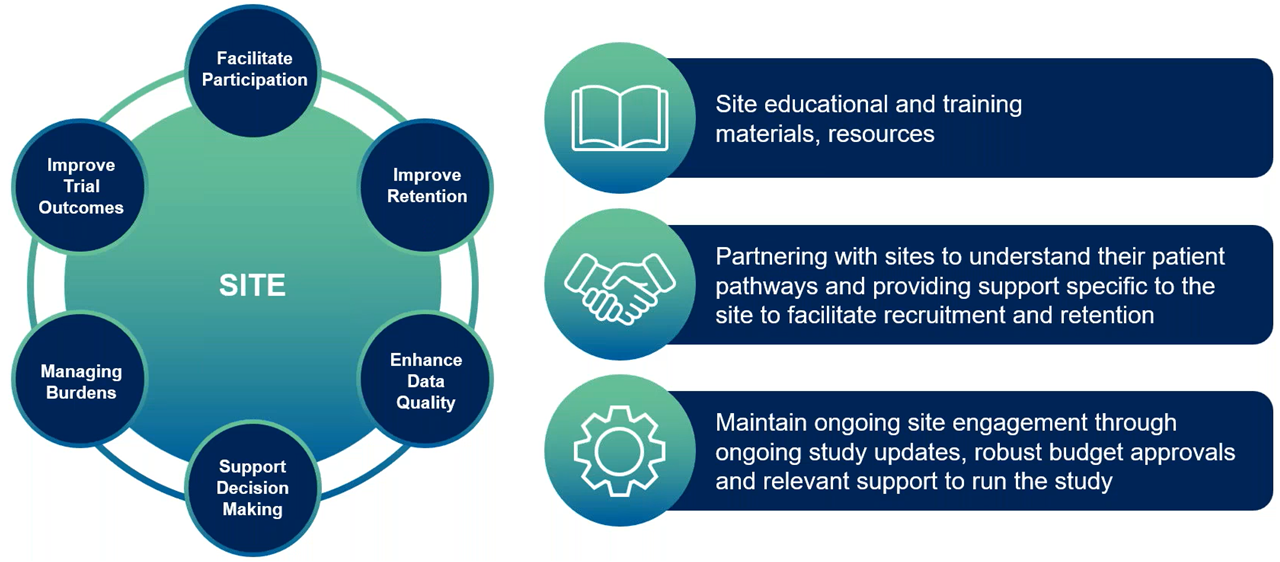
Figure 3. Methods to empower sites.
Once participants enroll, sites actively support retention through flexible scheduling, remote options and transportation assistance. Transparent communication about data privacy and expectations builds trust, while digital tools like apps and ePROs keep patients connected and engaged.
Campbell reinforced this approach: “Keeping patients motivated and connected to the study through multiple touchpoints — newsletters, apps and frequent communication — creates a support system so that patients can stay connected.”
Empowered sites also ensure that participants feel valued by offering personalized care, such as wellness items or mental health screenings for family members. These gestures acknowledge patients’ contributions and reduce the burdens associated with participation.
By equipping sites with the tools, training and support they need, psychiatry trials create a collaborative ecosystem that enhances retention, improves data quality and builds trust across all stakeholders.
Community-Based Recruitment and Diversity Efforts
Recruiting diverse patient populations is essential for psychiatry trials to reflect the demographics affected by mental health conditions. Trial teams address barriers like social stigma, cultural differences and limited access to psychiatric care by prioritizing community-based recruitment strategies and diversity.
Social stigma often prevents individuals from seeking help or participating in trials. Sponsors and CROs can combat this by engaging local communities through partnerships with mental health organizations, advocacy groups and support networks.
“Developing those relationships within the community so that you are not dropping in just for your clinical research study, but you have ongoing patient community engagement, is important.”
— Miaesha Campbell
Culturally sensitive recruitment materials, available in multiple languages, and collaboration with diverse healthcare providers ensure that messaging resonates across different communities. Trial teams can also partner with grassroots initiatives like the UK’s Men’s Sheds Association, which provides safe spaces for men to discuss mental health informally, reducing stigma and promoting trial participation.
Furthermore, by working directly with primary care physicians, general practitioners and community clinics, psychiatry trials can reach potential participants earlier and more effectively. Establishing referral pathways with primary care providers allows trials to connect with patients who may not have otherwise considered a psychiatric trial. Additionally, partnerships with local community health centers enable trials to reach underserved patients who may face barriers to accessing specialized mental health services.
To further support diversity, trials address financial and logistical barriers by offering travel reimbursements, flexible scheduling and remote participation options. These accommodations make trials more accessible and appealing, demonstrating respect for participants’ needs.
Reflecting on these efforts, Dr. Vornov stated, “Meeting the patient where they are means using community-based recruitment, not just mass advertising or databases, and seeking diverse populations. Once patients join, we must make it easy for them to participate, support their compliance and listen to their experiences to improve future trials.”
By embedding community engagement and cultural awareness into recruitment strategies, psychiatry trials improve inclusivity and generate data that better represents the populations affected by mental health disorders.
As the experts from Medpace discussed in the recent webinar, through comprehensive patient education, robust site support and a commitment to diversity, psychiatry trials can produce meaningful results that advance mental health treatments globally.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN