As many as 4.9 million Americans have moderate-to-severe hearing loss in one ear, also known as single-sided deafness (SSD). Existing hearing devices don’t work for everyone, leaving many individuals affected by SSD or asymmetric hearing loss (AHL) frustrated with carrying everyday conversations.
But now, recent US Food and Drug Administration (FDA) approvals of a cochlear implant system by the global tech company, MED-EL, will give these individuals a new option to improve their hearing.
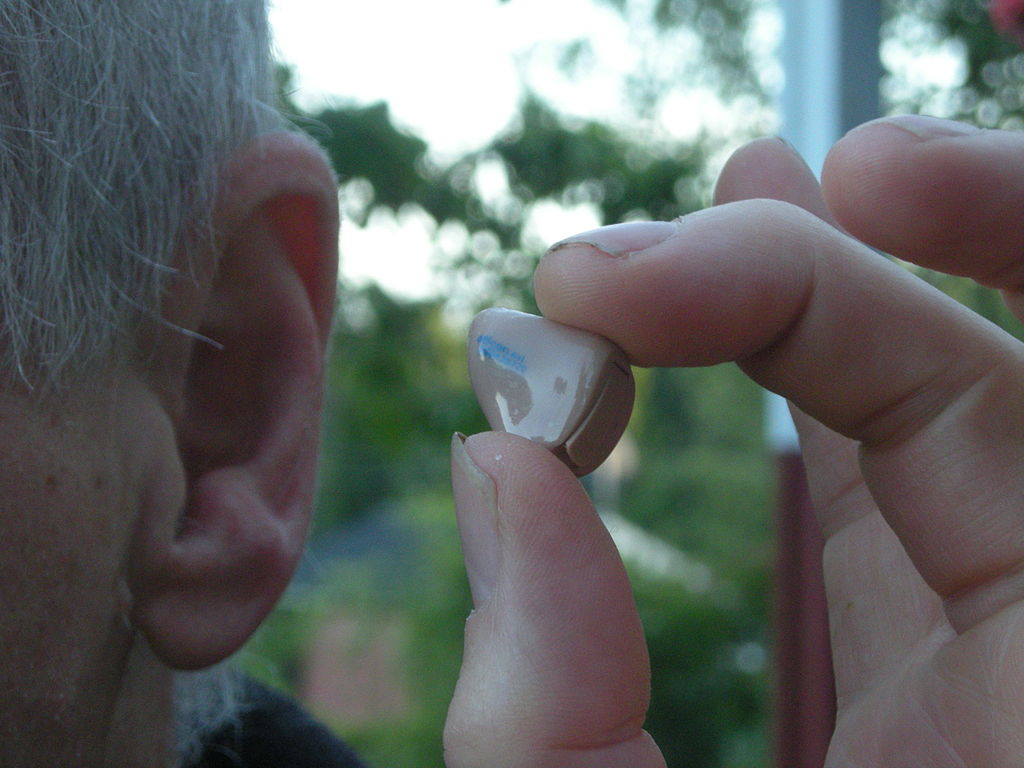
It might come as a surprise to learn how much single-sided hearing loss can affect a person’s quality of life. Normally, we rely on both ears to improve hearing quality, locate the source of a sound and distinguish different sound frequencies and intensities. But following a viral infection, trauma to the inner ear, Meniere’s Disease (or perhaps an unknown cause), individuals who develop SSD or AHL lose a lot of these abilities. They may also experience something known as the head shadow effect, where the head acts as a “barrier” to sounds going from the damaged ear to the healthy ear.
Because the extent of damage differs between patients, the type of hearing device they will benefit from will differ as well.
MED-EL will offer its cochlear implant system known as SYNCHRONY to individuals with profound hearing loss in one ear and normal to severe hearing loss in the other ear. The device comprises a small titanium implant and flexible electrode arrays to help convert sounds into electrical signals which are carried directly to the brain. Cochlear implants work differently compared to hearing aids which function to amplify sound, and bone conduction devices which cannot restore sound localization.
The approval was based on a University of North Carolina at Chapel Hill study conducted on 40 adults with SSD or AHL. All 40 participants reported improvements in their ability to locate sounds around them, to understand speech in a noisy environment and to carry conversations after one year with the MED-EL cochlear implant.
“It was gratifying to be able to demonstrate the efficacy of MED-EL’s cochlear implants to help patients restore a sense of sound that they had been missing,” said Dr. Margaret T. Dillon, Associate Professor at the University of North Carolina School of Medicine and lead researcher of the study.
The Austrian company also offers middle ear implants, electric acoustic stimulation devices, bone conduction systems and various signals processors to pair with each device.
In recent years, the hearing aid industry has seen major shifts in technology and regulations. Device manufacturers like Bose Corporation are releasing ‘self-fitting’ products which enable users to fit, program and control a hearing aid without assistance from a healthcare provider. Amendments made to the Federal Food, Drug and Cosmetic Act in 2017 will affect upcoming regulations on the sale of over-the-counter hearing aids, which are currently unavailable to consumers. Cochlear implants and middle ear implants will continue to be regulated as medical devices, requiring premarket approval before marketing.
Technological progress, changes to regulations plus the approval of the MED-EL SYNCHRONY cochlear implant will pave the way for better hearing options among individuals with hearing loss.
*Editor’s Note: An earlier version of this article mentioned that the latest audio processor, SONNET 2, and fitting software, MAESTRO 8, were unveiled earlier this month. This is not accurate for the US market in which the FDA approved the single-sided deafness indication.








Join or login to leave a comment
JOIN LOGIN