After declaring the monkeypox (mpox) virus a public health emergency of international concern in August, the World Health Organization (WHO) has approved the world’s first monkeypox test for emergency use.
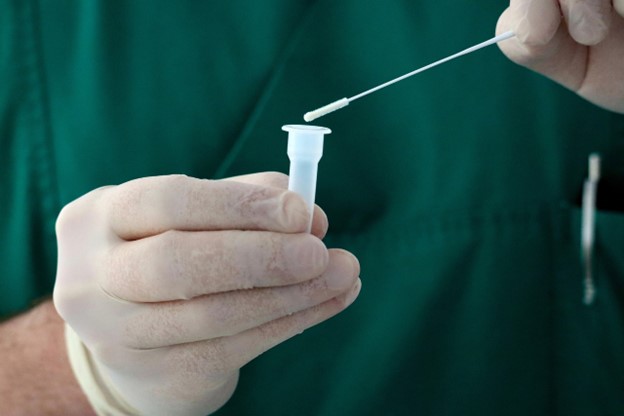
The approval was awarded to Abbott’s Alinity m MPXV assay, the first mpox in vitro diagnostic (IVD) listed under the WHO’s Emergency Use Listing (EUL) procedure.
The Alinity m MPXV assay is a real-time PCR test designed to detect mpox virus (clade I/II) DNA from swabs of human skin lesions. It is intended for use by trained clinical laboratory professionals skilled in PCR techniques and IVD procedures. By identifying DNA from pustular or vesicular rash samples, the test allows laboratory and healthcare workers to confirm suspected mpox cases quickly and accurately.
The approval for the assay will be pivotal in expanding global diagnostic capacity and access in countries facing mpox outbreaks where the need for quick and accurate testing has risen sharply, said the WHO. Early diagnosis of mpox enables timely treatment, care and control of the virus.
According to the WHO, limited testing capacity and delays in confirming mpox cases continue to challenge Africa, exacerbating the spread of the virus. In 2024, the region has so far reported over 30,000 suspected cases, with the highest numbers occurring in the Democratic Republic of the Congo (DRC), Burundi and Nigeria. In the DRC, only 37 percent of the plausible cases were confirmed through testing, highlighting the urgent need for improved diagnostic infrastructure. Mpox has led to at least 635 deaths in the DRC this year.
Mpox has been declared a public health emergency for the second time in two years, first in 2022 and now in 2024.
Mpox is a viral zoonotic disease that was first discovered in monkeys in the 1950s. The mpox virus is a species of the genus Orthopoxvirus. While previously considered a rare disease limited to remote parts of Central and West Africa, recent years have seen a rise in outbreaks outside of these regions, prompting global health authorities to elevate their surveillance and response efforts.
XTALKS WEBINAR: Medical Device Safety: Navigating Regulations and Standards to Determine Non-clinical Requirements
Live and On-Demand: Wednesday, November 6, 2024, at 1pm EST (10am PST)
Register for this free webinar to learn how the intended use, technological characteristics and risk classification of a medical device relates to standards.
There are currently two strains of mpox in circulation and spreading — the clade I variant, which is endemic in parts of West and Central Africa, and the new, more transmissible strain, clade Ib, that has caused more global concern.
Outside the DRC and other countries in Africa, Sweden, Thailand and India have confirmed cases of the clade Ib type of the virus.
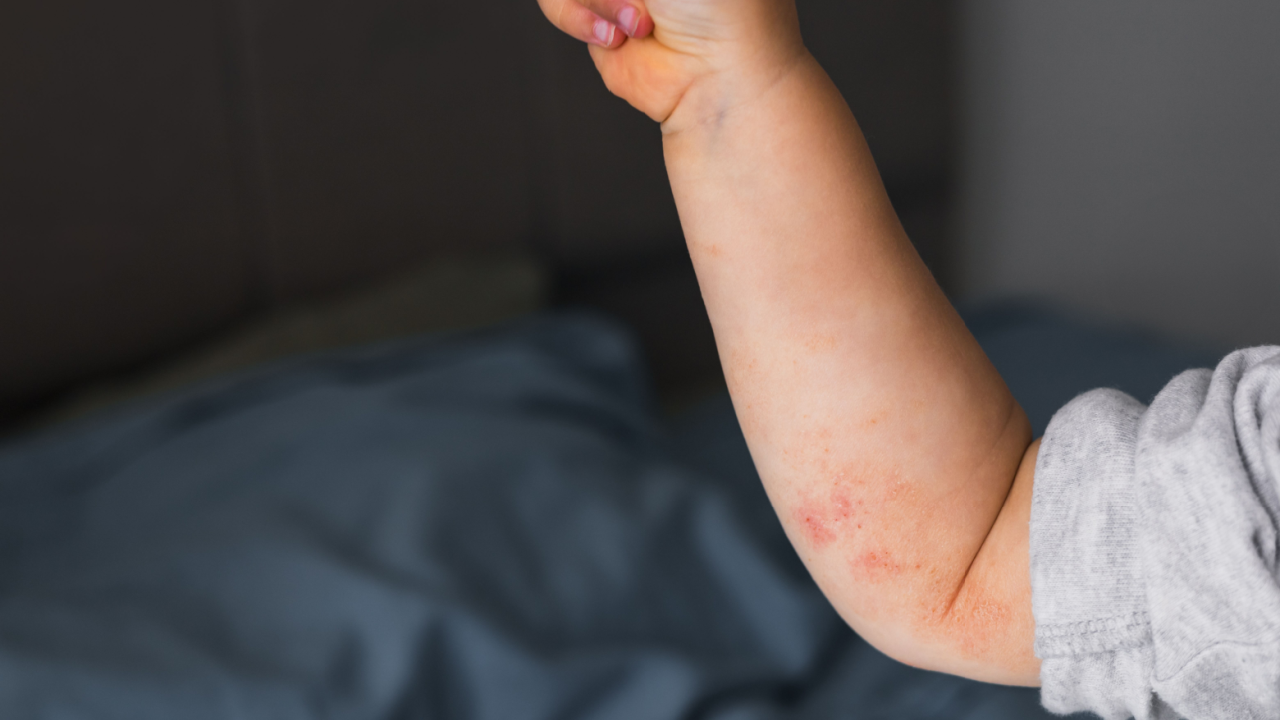
While mpox shares similarities with smallpox, which is caused by the variola virus that is part of the Orthopoxvirus genus like the mpox virus, mpox tends to be less severe and leads to much fewer deaths. Symptoms of mpox include fever, rashes, swollen lymph nodes and in some cases, complications that can lead to serious health risks.
In the statement announcing approval of the mpox test, WHO Access to Medicines and Health Products assistant director-general Dr Yukiko Nakatani said, “This first mpox diagnostic test listed under the Emergency Use Listing procedure represents a significant milestone in expanding testing availability in affected countries. Increasing access to quality-assured medical products is central to our efforts in assisting countries to contain the spread of the virus and protect their people, especially in underserved regions.”
Prior to the WHO-approved test, diagnosing mpox relied heavily on clinical assessment and laboratory testing with limited global availability. Given the virus’s transmission between humans, animals and through contaminated materials, rapid and accurate detection has become critical to controlling outbreaks. Moreover, symptoms of mpox can resemble other diseases, such as chickenpox or measles, making diagnostic precision essential.
The approval of the mpox test has wide-reaching implications for global health as it enables countries to improve disease surveillance, monitor outbreaks more effectively and prevent uncontrolled transmission. For healthcare workers, it reduces the burden of uncertain diagnoses and allows for targeted treatment and care. Early diagnosis also means patients can be isolated and treated promptly, reducing the risk of secondary transmission to family members and caregivers.
While the approval of the mpox test is a significant milestone, global health advocates are emphasizing the need for equitable distribution. The WHO has highlighted the importance of making the test accessible, particularly in low- and middle-income countries where healthcare resources are limited and the potential for outbreaks is higher.
At the end of August, the WHO also encouraged manufacturers of mpox IVDs to submit expressions of interest for EUL, acknowledging the urgent need to enhance global testing capacities as the virus continued to spread. The WHO explains that the EUL process evaluates the quality, safety and performance of essential health products, such as diagnostic tests, to help procurement agencies and WHO Member States make informed decisions for emergency procurement within a limited timeframe.
WHO said it has received three more submissions for EUL evaluation while discussions are currently ongoing with other manufacturers. This will support countries that don’t have any approved tests yet, enabling them to obtain them through United Nations (UN) agencies and other procurement partners.
If you want your company to be featured on Xtalks.com, please email [email protected].









Join or login to leave a comment
JOIN LOGIN