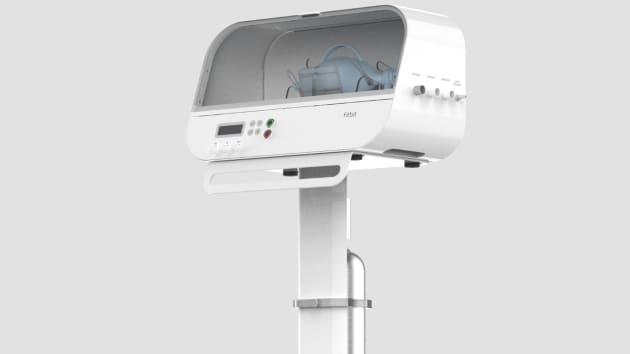
Xtalks has learned that renowned maker of health wearable devices and fitness activity trackers, Fitbit, is working to design and manufacture custom, emergency-use ventilators for the treatment of COVID-19 patients that require respiratory support. The company plans to submit its designs for the machine to the US Food and Drug Administration (FDA) for emergency use authorization in the next several days.
The ventilator will have specialized features not present in traditional ventilators used in hospitals, and overall, will be far more advanced than ventilators being produced by various manufacturers as part of emergency response measures during the pandemic.
The more sophisticated ventilators will include features that can add heat and humidity to the air in hospital environments including intensive care units. The ventilator also has the ability to vary the amount of pressure delivered in order to help keep the lungs of patients more fully inflated following exhalation.
In an interview with CNBC, James Park, CEO of Fitbit, said that the device “would be somewhat more advanced but still come in at a lower price point compared to the premium, durable ventilators designed for long-term hospital use.”
Fitbit collaborated on a prototype for the device with physicians at Oregon Health & Science University, Massachusetts General Hospital and Brigham and Women’s Hospital. It plans to shift its supply chain to begin manufacturing of the ventilators once it receives the FDA approval.
Related: CT Imaging May Be a Preferred Diagnostic Test for COVID-19
“I think one of the advantages for us is that we have the infrastructure and manufacturing capability,” Park said. “We already make 10 million (wearable) devices per year, and we plan to leverage that to make deliver product at whatever volumes are needed.” He added that, “there was a lot of concern about the shortage of ventilators and we realized we had expertise already around the supply chain.”
The company has proposed to work with an existing vendor in Taiwan in order to rapidly scale up production of the ventilators once FDA approval is received.
Ventilator shortages have posed to be a significant concern in countries around the world during the COVID-19 pandemic, including the pandemic epicenters of New York State in the US and Italy. While COVID-19 numbers are leveling off in many parts of North America and Europe, secondary waves of the infection are expected in the coming months, particularly in the upcoming fall/winter flu season.
Moreover, as cities and countries begin lifting lockdown measures and reopening, cases could begin to surge again, increasing the demand for ventilators and other medical supplies.
COVID-19 numbers are currently increasing in many parts of the world including India, Brazil and other Latin American countries, which had previously been reporting low numbers of the infection. All of this warrants the need for keeping up stocks of medical supplies and equipment.
Although some places in the US are currently reporting a surplus of ventilators, this could quickly change given how rapidly the pandemic is evolving. Hence, there are continued calls for making more ventilators, with companies like Fitbit redirecting and repurposing their supply chains for their production. Xtalks will keep a lookout for the latest developments on this story and more.






Join or login to leave a comment
JOIN LOGIN