In a recent webinar, Jess Robinson, Director of Study Start-up at GSK, highlighted the importance of aligning people, processes, and technology when embarking on a study start-up transformation journey.
A deep dive into cycle time metrics for key start-up milestones was the catalyst for change at GSK. After benchmarking their performance against industry standards, they realized they needed to change how they work to optimize clinical delivery.
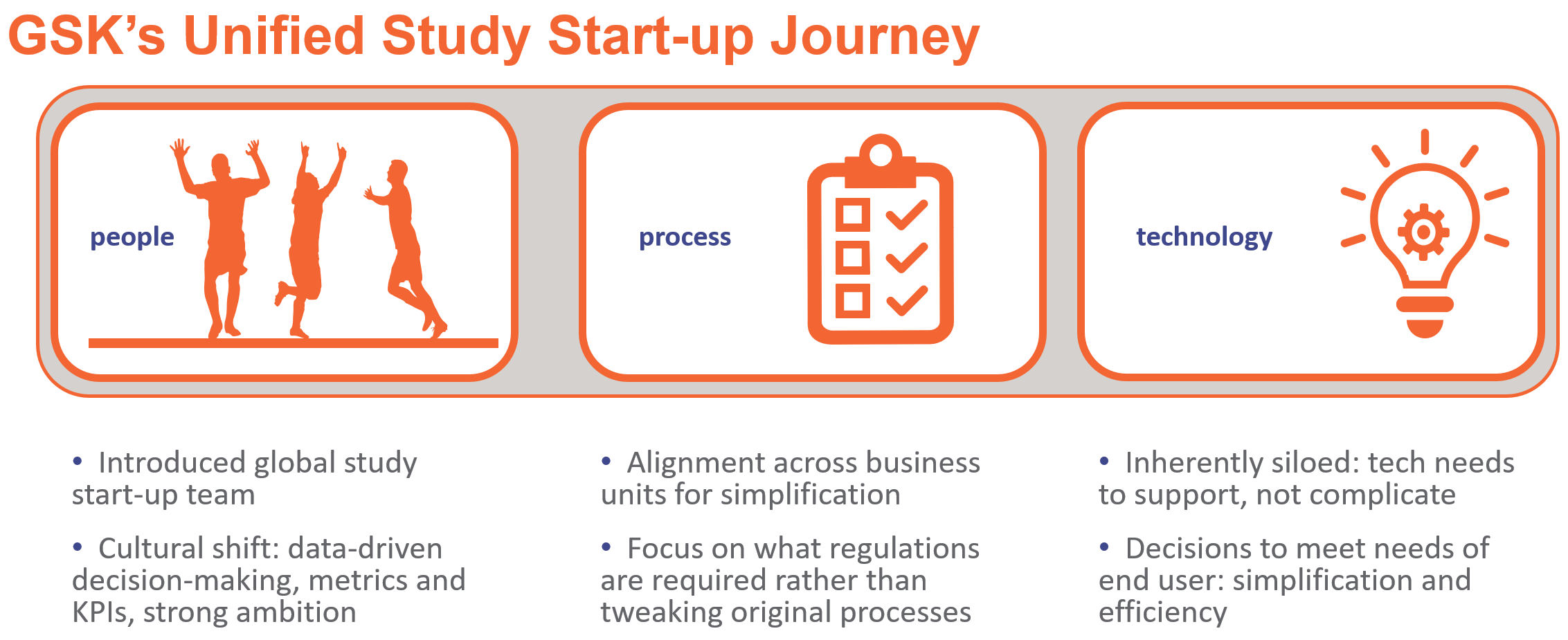
GSK understood implementing new technology in a silo without considering the impact on people and processes would not be successful, so they incorporated all three pillars into their Vault Clinical deployment to work smarter, better, and faster.
PROCESS
Rather than adjusting old processes to fit a new system, GSK’s global study start-up team partnered with business owners to re-engineer processes to fit the new ways of working. With a focus on improving efficiencies through simplification, the technology and business teams collaborated to establish project objectives, define goals, and gain alignment.
PEOPLE
Because a transformation project requires significant internal resource investment, GSK developed strategies that mapped to key activities such as engagement with stakeholders, and training and communication plans to ensure business readiness. They identified change champions throughout the organization to evangelize the new workstreams, drive accountability across business owners, and assist study country teams with the technology rollout.
TECHNOLOGY
Eliminating siloed workflows, transitioning to data-driven decision-making, and simplifying the experience to improve productivity for end-users and sites were key drivers for GSK that shaped the decision criteria for the new technology. A streamlined user interface, on-screen navigation cues, and visibility to KPIs and metrics for different functional areas greatly improved technology adoption.
Fostering change across the people, process, and technology pillars has had a meaningful impact on GSK’s operations and ability to accelerate study delivery. Register for the Veeva R&D and Quality Summit on October 13-14 to hear more about GSK’s study start-up journey and a deep dive into how they prepared the business for study start-up change.
Stay tuned for the next blog in the series, where we’ll explore unified vs. integrated study start-up technology and the value for GSK in more detail.
This blog post was originally published by Veeva.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.









Join or login to leave a comment
JOIN LOGIN