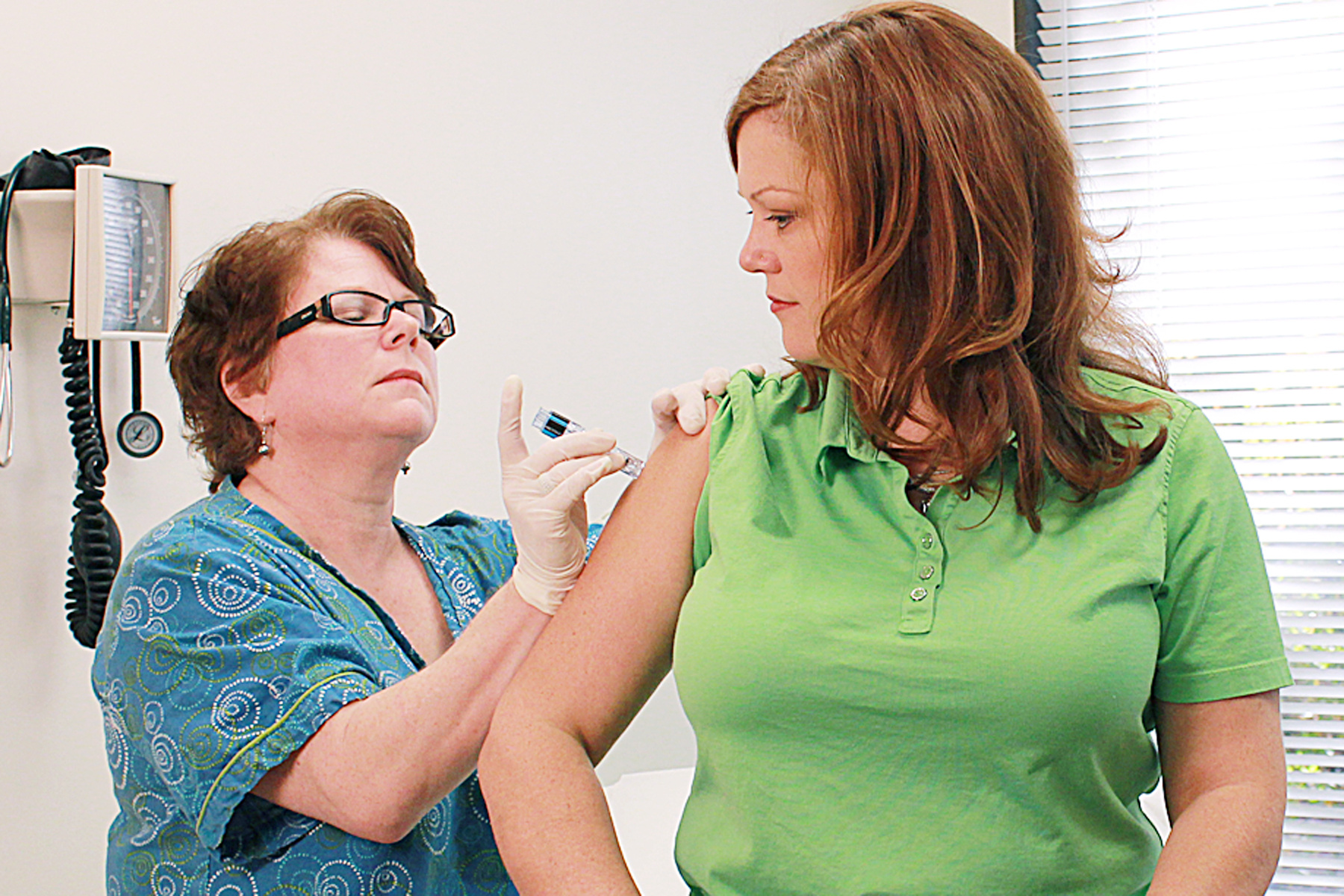
It’s understandable that parents of children who have experienced a reaction after being vaccinated would be hesitant to get them vaccinated again, but a study published in The Pediatric Infectious Disease Journal suggests that they’re unlikely to experience further adverse events. The study comes at a time when public trust in the safety of vaccines – particularly in children – has been questioned, largely due to the unsubstantiated link between vaccines and autism.
“Most patients with a history of mild or moderate adverse events following immunization [AEFI] can be safely reimmunized,” wrote Dr. Gaston De Serres and his colleagues from Laval University in their publication. “Additional studies are needed in patients with serious AEFIs who are less likely to be reimmunized.”
While there is still a risk that children could experience recurrent AEFIs after being reimmunized with the same vaccine, the researchers found in the current study that the overall risk was low. This means that children whose adverse reactions were only mild to moderate after the initial vaccine can safely be given another dose.
In the US, the Vaccine Adverse Event Reporting System (VAERS) is used to track AEFIs, with a similar system being used in Quebec, Canada where the current study was conducted. Healthcare professionals are required to report patients’ reactions to immunization to this system, particularly when they are deemed to be “unusual or severe.”
Using this database, De Serres and his colleagues looked at 5,600 reports of AEFIs which were logged between 1998 and 2016. Reports included a range of vaccines – half of which elicited allergic reactions – however the researchers excluded adverse events associated with the flu vaccine as the strains used in the shot aren’t consistent from year-to-year.
In all, 1,731 of the 5,600 patient records included follow-up date, with 78 percent of these patients being re-vaccinated, many of whom were under the age of 24 months. Just 16 percent of those who originally experienced AEFIs had a recurrent reaction after being re-vaccinated.
What’s more, 80 percent of patients who did experience a recurrent AEFI had no more serious a reaction than their initial response. These events were less likely in children under the age of two – a time when parents often express concern over the number of vaccines their children are getting.
Patients with limb swelling and other local reactions after their first vaccination were at the highest risk of experiencing subsequent reactions, but anaphylaxis was uncommon upon re-vaccination. Interestingly, different vaccines were not associated with different recurrence rates of AEFIs.
While fewer children who initially experienced severe AEFIs were less likely to be re-vaccinated, they showed a lower risk of recurrence when compared to children who only had a mild or moderate reaction to a vaccine.
The findings of this large study are valuable to medical professionals as little data on the recurrence of AEFIs existed before. De Serres and colleagues say that increased follow-up reporting on the adverse events database could help with further research on the safety of re-vaccination after AEFIs.





Join or login to leave a comment
JOIN LOGIN