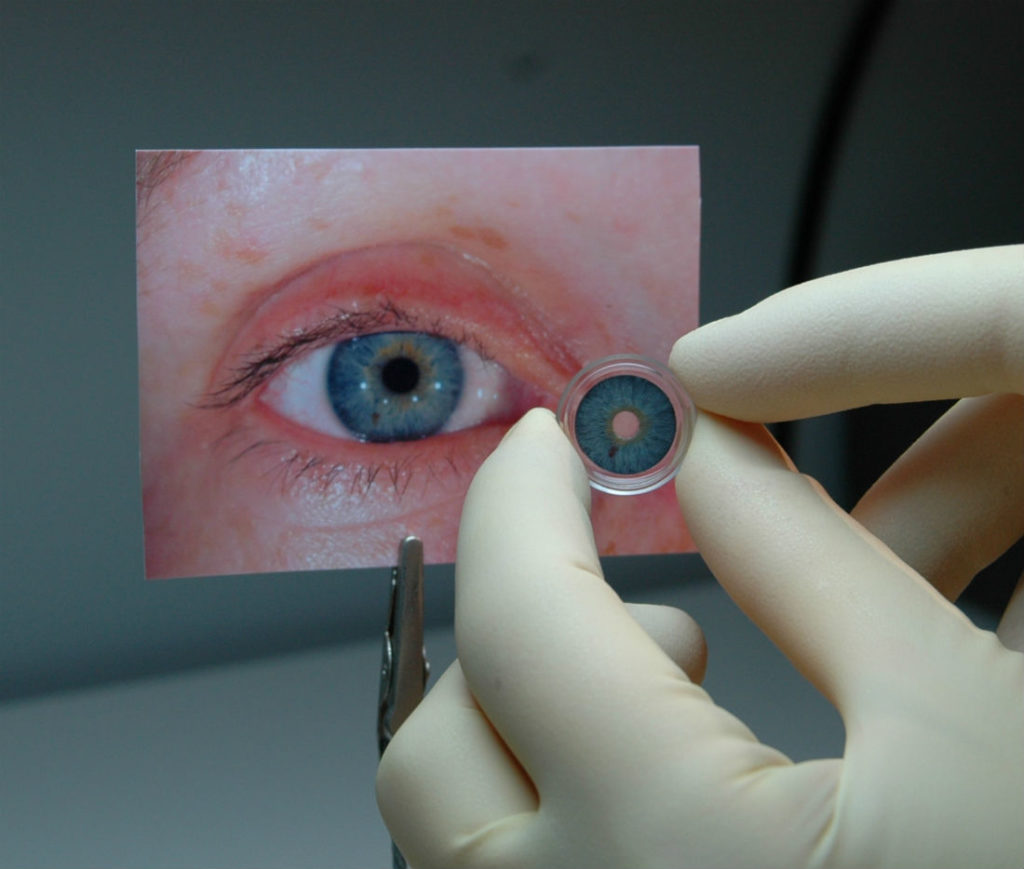
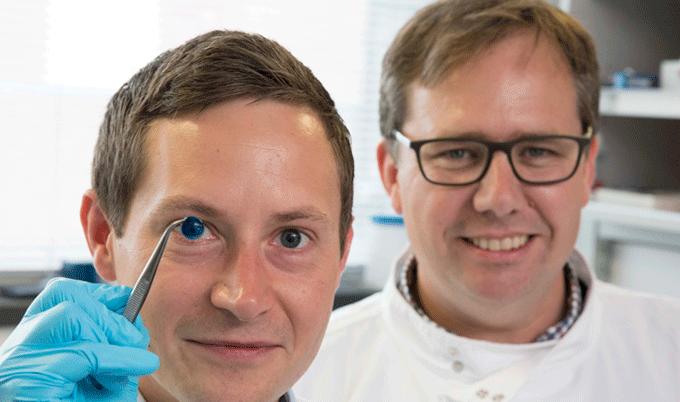
The first prosthetic iris has been approved by the FDA to treat patients whose eyes have been damaged due to a congenital condition or traumatic injury. HumanOptics’ CustomFlex Artificial Iris can be implanted into a patient to control the amount of light that enters the eye.
A rare genetic disorder known as congenital aniridia is one cause of a missing or partially-formed iris. Affecting between 1 in 50,000 and 1 in 100,000 individuals in the US, congenital aniridia can cause vision problems and increase a patient’s sensitivity to light exposure.
Currently, many patients with aniridia may require glasses to improve their vision. Because of their increased light sensitivity, tinted lenses may help to reduce this symptom.
“Patients with iris defects may experience severe vision problems, as well as dissatisfaction with the appearance of their eye,” said Dr. Malvina Eydelman, director of the Division of Ophthalmic, and Ear, Nose and Throat Devices at the FDA’s Center for Devices and Radiological Health. “Today’s approval of the first artificial iris provides a novel method to treat iris defects that reduces sensitivity to bright light and glare. It also improves the cosmetic appearance of the eye in patients with aniridia.”
Composed of flexible, medical-grade silicone, the CustomFlex Artificial Iris is custom made for each patient based on the individual’s eye size and colour. Patients with albinism as well as those who have had their iris removed due to melanoma are also eligible to receive the CustomFlex Artificial Iris.
The prosthetic device is folded and inserted into the eye through a small incision. After it’s unfolded within the eye, the artificial iris may be held in place by small sutures or by the eye’s natural, anatomical structures.
HumanOptics was granted Breakthrough Device designation for the CustomFlex Artificial Iris which accelerated the agency’s review of the device and provided the company with additional device development support. The device was passed through the FDA’s premarket approval pathway which is a stringent review process for devices considered to be high-risk.
A clinical trial involving 389 adults and children with varying levels of iris defects was conducted to assess the safety and efficacy of the prosthetic device. Over 70 percent of patients in the study reported improvements in their health-related quality of life after the procedure and found they were less sensitive to light and glare. Encouragingly, 94 percent of those patients reported that they were satisfied with the appearance of the artificial iris.
While the study boasted a high success rates, some adverse events were reported that were related to implantation of the device. Increased intraocular pressure, device dislocation and inflammation of the iris were all complications that arose as a result of the prosthetic.





Join or login to leave a comment
JOIN LOGIN