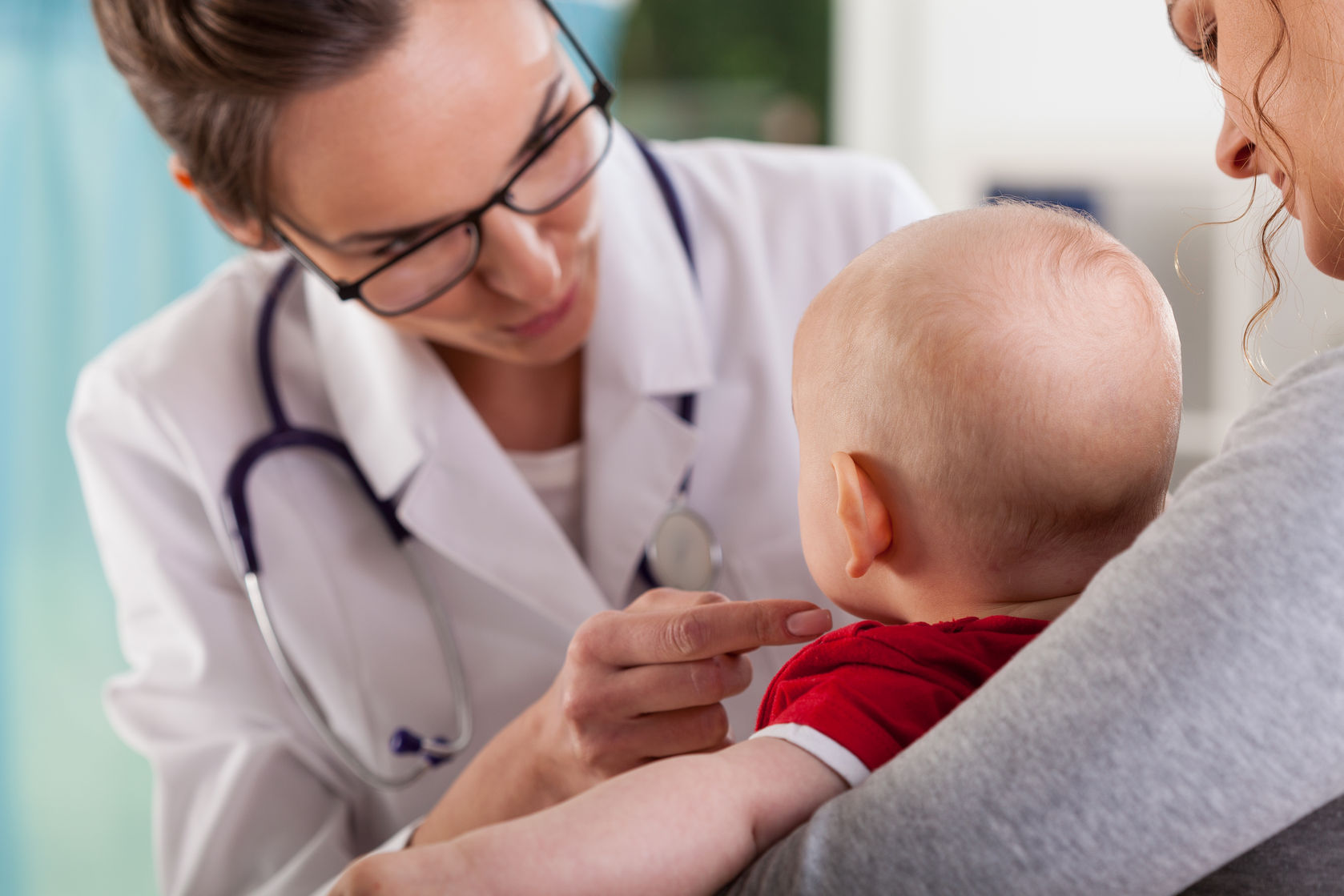
UK-based pharmaceutical company Mallinckrodt has announced that the first pediatric patient with Duchenne muscular dystrophy has been enrolled into the company’s Phase II trial of their investigational drug MNK-1411. The study will seek to test the safety and efficacy of MNK-1411 (also referred to as cosyntropin or tetracosactide) in patients between the ages of four and eight.
“We are very excited Mallinckrodt has chosen to study how this investigational drug, MNK-1411, might help in treating Duchenne Muscular Dystrophy,” said Pat Furlong, president and CEO, Parent Project Muscular Dystrophy. “In the fight to end Duchenne muscular dystrophy, our community welcomes partners dedicated to this goal.”
The clinical trial – dubbed the BRAVE study – will aim to enroll about 130 pediatric patients diagnosed with Duchenne muscular dystrophy. In order to qualify for participation in the trial, patients will need to have a confirmed complete dystrophin protein deficiency or a confirmed mutation in the dystrophin gene. Patients must also display clinical symptoms consistent with Duchenne muscular dystrophy, including muscle weakness and impaired mobility.
The randomized, placebo-controlled trial will assess the safety and efficacy of two weight-based doses of MNK-1411 administered via subcutaneous injection twice a week over a 24-week study period. An open-label extension study will follow the trial to give patients who were randomized to the placebo group an opportunity to get the drug treatment.
Functional improvements using timed function tests – including a 10-meter walk/run, 4-stair climb and rise from floor, and the NorthStar Ambulatory Assessment – will be used as outcome measures for the efficacy of the drug.
As Duchenne muscular dystrophy is a rare disease which affects about one in every 3,500 male births around the world, the study coordinators may face challenges when trying to recruit a significant number of patients in which to test the drug. However, the company’s enrollment target of 130 boys isn’t far off the number of patients participating in other Duchenne muscular dystrophy trials registered on ClinicalTrials.gov.
“Mallinckrodt is focused on meeting the unmet medical needs of patients, particularly those with rare diseases like DMD,” said Dr. Steven Romano, executive vice president and chief scientific officer at Mallinckrodt. “We are pleased to enroll the first patient in our Phase 2 clinical trial of investigational drug MNK-1411 in boys with DMD. We are also excited by the recent EMA designation of Orphan Drug status as confirmation of the potential benefit of this investigational therapy for European patients.”
The EMA’s decision to grant Orphan Medicinal Product designation for MNK-1411 could offer Mallinckrodt a whole host of incentives, from fee exemptions to a prolonged period of market exclusivity.
For now, there are a limited number of drugs approved by regulators to treat Duchenne muscular dystrophy. In the US, PTC Therapeutics’ Emflaza and Sarepta Therapeutics’ Exondys 51 have been approved by the FDA to treat patients with the disease, however the EMA has yet to approve either drug for this indication.









Join or login to leave a comment
JOIN LOGIN