Metabolic dysfunction-associated steatohepatitis (MASH), previously known as non-alcoholic steatohepatitis (NASH), is a growing health concern. In the US, MASH affects an estimated three to six percent of the population.
This chronic liver disease results from fat accumulation that leads to liver cell damage and inflammation. It is often associated with obesity, type 2 diabetes and cardiovascular risks.
MASH is a more advanced form of metabolic dysfunction-associated steatotic liver disease (MASLD) and, if left untreated, MASH can progress to cirrhosis, liver failure and an increased risk of hepatocellular carcinoma.
The prevalence of MASH in the US is expected to rise, with cases projected to increase by 63 percent from 2015 to 2030, growing from 16.5 million to 27 million. As one of the leading causes of liver-related morbidity and a potential precursor to liver cancer, MASH is also expected to increase demand for liver transplants.
The surge in MASH cases stresses the need for early diagnosis, timely intervention and precise, reliable methodologies in clinical trials to evaluate new therapies effectively.
“MASH represents a growing major health concern, and depending on the projection you see, it is due to impact quite a large patient population,” said Jenson Ruiz-Garcia, MSc, Clinical Trial Management at Medpace, emphasizing the scale of the issue.
In a recent webinar led by Ruiz-Garcia and his colleagues at Medpace—Dr. Salvatore Zabbatino, MD, Senior Director, Core Laboratories; Dr. Robert McGee, MD, PhD, Senior Medical Director, Central Laboratories; and Dr. Nicola Owen, PhD, MBBS, Medical Director—they shared insights into the operational and medical strategies that support the design and conduct of MASH clinical trials.
Read on to gain insights from Medpace experts about advancements in imaging, histology and innovative techniques for MASH clinical trials. Learn how these approaches enhance data collection, sample standardization and patient-centered study designs.

Photo courtesy of Medpace.
Endpoint Determination: The Role of Liver Biopsy and Regulatory Guidance
In MASH clinical trials, determining accurate and reliable endpoints is essential to determine a therapy’s efficacy and safety.
Liver biopsy remains the “gold standard” for evaluating key histological features, such as steatosis, hepatocellular ballooning, lobular inflammation and fibrosis. These markers are used to understand disease progression and play a critical role in meeting regulatory standards and securing marketing authorization.
Regulatory Standards for MASH Studies
Both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have provided detailed guidance for drug development in MASH, highlighting that liver biopsy is necessary for determining disease progression as part of composite endpoints.
These composite endpoints often integrate both histological and clinical features, such as reduction in liver fat or inflammation and improvement in patient quality of life, to create a holistic view of treatment impact. Liver biopsy, therefore, becomes indispensable in capturing changes that might otherwise go undetected in imaging or clinical assessments alone.
“The FDA and EMA guidance in terms of drug development for endpoints in MASH outcomes is very key that we do require liver biopsy for disease progression, and it encompasses as part of their composite endpoints.”
— Jenson Ruiz-Garcia
Liver biopsies are necessary for assessing these endpoint markers and are also needed for establishing participant eligibility. This is especially relevant as many MASH patients present with multiple comorbidities, such as cardiovascular disease or diabetes, which can complicate trial participation.
Accurate assessment via biopsy helps ensure that patients who enter the study meet the histological criteria required for inclusion, which is needed for gathering reliable study outcomes.
Regulatory Encouragement of Non-Invasive Technologies (NITs)
While regulatory bodies recognize the challenges associated with invasive liver biopsies, particularly concerning patient recruitment and retention, they acknowledge the current limitations of non-invasive technologies (NITs) in MASH clinical trials.
“At the moment, liver biopsy is here to stay, although we do acknowledge that there is increasing use of non-invasive tests, but so far, they have not demonstrated that sensitivity and specificity, although they appear to have an increasing role in perhaps finding those subjects that might have eligible biopsies.”
— Nicola Owen
NITs are still not adequately validated to replace histology as intermediate endpoints in MASH studies. Despite advancements in imaging, biomarker identification and magnetic resonance imaging-proton density fat fraction (MRI-PDFF) methods for liver fat quantification, these technologies have yet to demonstrate the specificity and sensitivity necessary to replace biopsy reliably.
According to Ruiz-Garcia, regulators are encouraging ongoing research and validation of these NITs, which are likely to play a complementary role alongside biopsy, rather than serving as standalone endpoints for the near future.
Patient-Centric Approaches to Managing Biopsy Burden
Patient-centric trial designs are essential in MASH clinical trials as the physical discomfort and anxiety associated with liver biopsies can lead to high dropout rates, potentially jeopardizing endpoint integrity.
Ruiz-Garcia emphasized that patient education, awareness and a feeling of participating in the clinical trial journey are key in preparing patients and sites for biopsy eligibility and preserving critical endpoints.
Effective trial strategies include minimizing biopsy frequency without compromising endpoint quality or regulatory standards. Educating participants on the purpose and safety of biopsies, setting realistic expectations and informing them about the risk of complications (with around 2.5 percent of MASH patients reporting biopsy complications) can alleviate some of the fears around these procedures.
Additionally, regular updates during the study reinforce patients’ understanding of their impact on MASH research. Trial teams often use supportive strategies like check-ins and clear explanations of how each biopsy supports study goals. This approach helps retain participants by fostering a sense of purpose and commitment, ultimately ensuring the study’s validity.
Optimizing MASH Clinical Trials Through Standardized Biopsy Handling and Sample Selection
Standardizing biopsy handling in MASH clinical trials plays a critical role. According to Dr. McGee, every step in the histology lab can affect the reliability of trial data and the interpretations made by MASH pathologists.
Over years of clinical trial experience, several “lessons learned” have shaped best practices, particularly in standardizing pre-analytical variables and selecting optimal biopsy sample types.
Importance of Pre-Analytical Standardization
Pre-analytical variables cover all procedures involved in handling liver biopsy samples from the point of collection to the start of analysis. These variables include sampling method, fixation details (such as type, volume, time and temperature of the fixative) and microtomy.

liver biopsy samples.
Photo courtesy of Medpace.
For MASH clinical trials, standardizing these elements can reduce variability in histological assessments and help ensure consistent data. Dr. McGee explained that these factors, if not uniform, can cause variability in staining quality, complicating reliable endpoint determination.
Two particularly important variables in the standardization process are fixation type and exposure time. For instance, 10% neutral buffered formalin is commonly used, but regional differences (such as higher formalin concentrations in Asia-Pacific areas) can affect staining characteristics. Similarly, formalin exposure time—ideally limited to six to 72 hours—must be controlled to preserve tissue integrity during shipping.
Dr. McGee noted that standardizing these variables is also important as AI tools begin to assist with fibrosis and collagen assessments, requiring a uniform sample presentation for best algorithm analysis.
Selecting the Best Sample Types for Reliable Data
Sample quality is another important consideration, as biopsy specimen type directly impacts the study’s outcomes. Dr. McGee outlined preferred biopsy types, beginning with the “gold standard”—the original uncut paraffin block, which retains the maximum amount of tissue (Figure 1).

Figure 1. Best sample types for a MASH study.
If an original block is not available, a “historic cut paraffin block” from the site is the second-best option, though it may have less residual tissue. Communication with the pathology lab about the specific needs of a MASH trial is crucial to ensure as much tissue is kept as possible for central review. In cases where paraffin blocks cannot be shared, unstained tissue sections can be sent instead, though these come with limitations in tissue volume and potential fragility during transit. Lastly, fresh wet tissue biopsies are the least preferred due to their vulnerability during shipping.
As Dr. McGee explained, adequate biopsy samples provide participants with “the best opportunity to get into the study,” reinforcing the need for careful coordination and sample handling.
Communication and Collaboration: Key to Biopsy Success
“The preparation and standardization of liver biopsy samples require good communication from the site to those involved in preparing and handling the sample.”
— Robert McGee
Clear communication between trial sites, pathology labs and imaging centers is essential to ensure the quality of biopsy samples for MASH trials.
When third-party imaging centers are involved, sites should communicate specific biopsy requirements, such as preferred needle gauge and core length, to avoid receiving insufficient samples.
Properly prepared samples with optimal tissue volume maximize the likelihood that histological features are captured, supporting accurate eligibility assessments and endpoint determinations.
Through these standards, MASH clinical trials can achieve greater consistency and reliability in histological data, aligning with regulatory expectations and making the way for accurate trial outcomes.
Addressing Variability in MASH Biopsy Slide Evaluation
Dr. Zabbatino emphasized that in MASH trials, standardized liver biopsy slide evaluation is essential for consistent, reliable data.
“Medpace Core Lab coordinates the review and the scoring of the slides for the purpose of eligibility, as well as for the efficacy and safety endpoints that are going to be used in these clinical trials,” explained Dr. Zabbatino.
A key advancement in MASH evaluation was the development of the NAFLD Activity Score (NAS), created by Dr. Kleiner and a team of pathologists from the NASH Clinical Research Network (CRN) and the National Institutes of Health (NIH) in 2005.
NAS is a semi-quantitative scoring system designed to assess change over time in liver disease, focusing on specific histological features rather than the speed of disease progression. Built from a dataset of 50 cases, including adult and pediatric samples, the NAS system uses hematoxylin and eosin (H&E) and trichrome stains to score critical MASH criteria, such as steatosis, lobular inflammation and ballooning.
The NAS scoring criteria provide a structured approach for evaluating disease severity. Steatosis, for example, is scored based on fat accumulation, from 0 (less than five percent of the slide) to 3 (more than two-thirds). Lobular inflammation is scored from 0 to 3 based on the presence of inflammatory foci in the liver tissue and ballooning is scored from 0 to 2 based on its prominence (Figure 2).
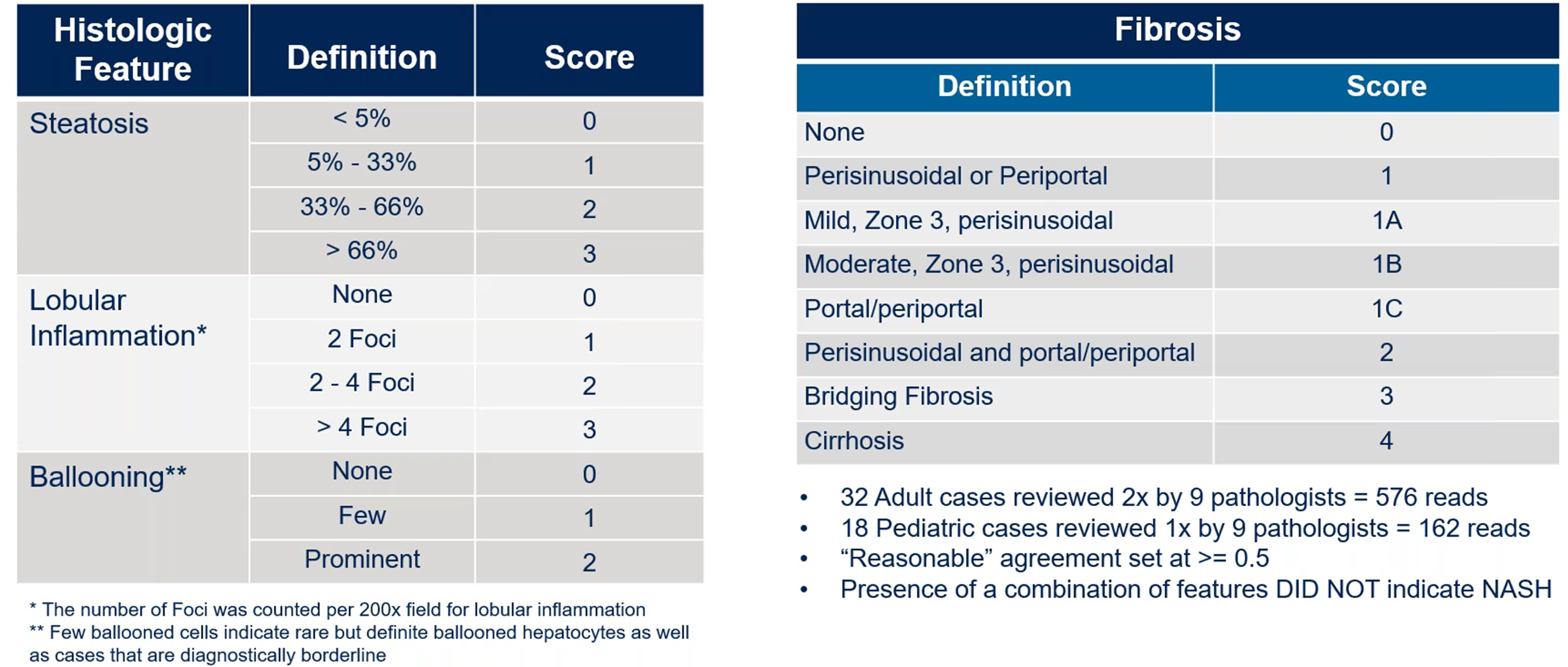
Figure 2. NAS scoring criteria. Adopted from Kleiner, David E., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005 41(6): 1313-1321.
In the context of NAS scoring and clinical pathology, kappa statistics measure the agreement between different pathologists (or “readers”) when scoring histological features in liver biopsies, such as steatosis, ballooning, inflammation and fibrosis. The kappa statistic ranges from -1 to 1, where values >0 indicate that agreement is greater than random chance, 0 suggests agreement is no better than random chance and <0 indicates that agreement is less than that expected by chance. To put it simply, a higher kappa score means better consistency among pathologists.
About 15 years later, Dr. Kleiner and colleagues re-evaluated these kappa metrics to assess any improvement in interobserver variability (Figure 3). Surprisingly, kappa scores remained largely unchanged, suggesting that there was not much change after 14 years of using the scoring system.
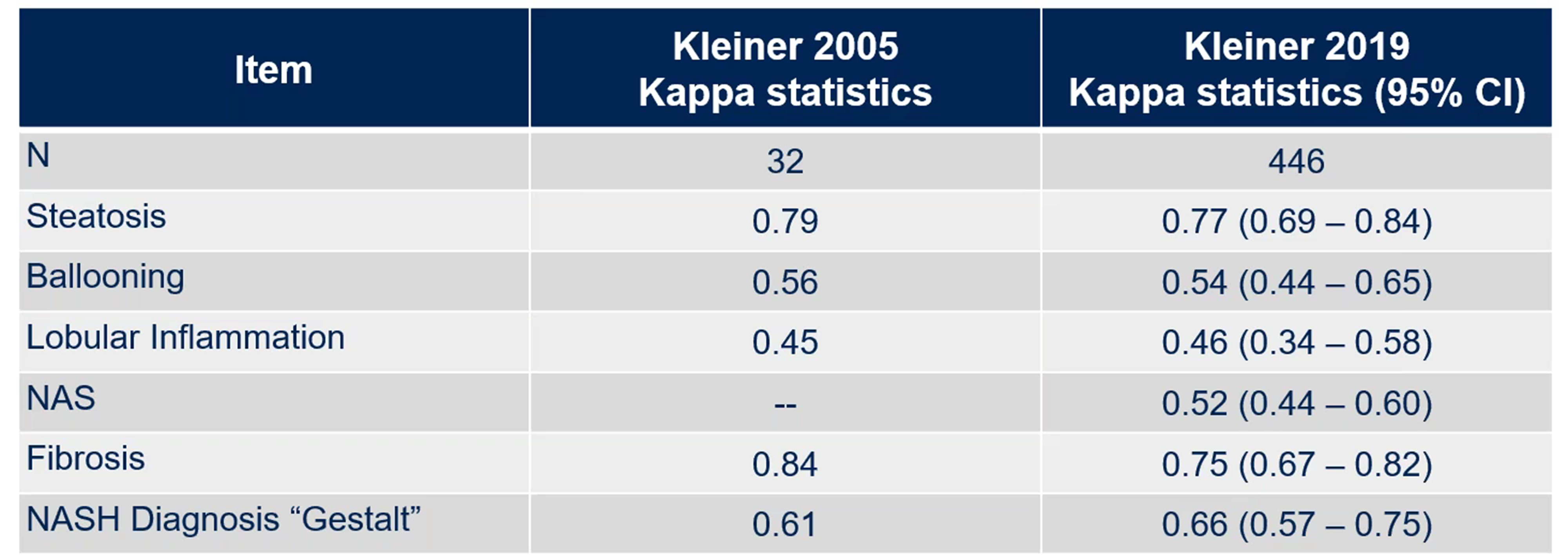
Figure 3. Assessing interobserver variability after 14 years of using the NAS scoring system. Adopted from Kleiner et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005 41(6): 1313-1321; and Kleiner et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA network open 2019 (2)10: e1912565.
In another study, two pathologists underwent intensive training with the aim of potentially reducing the variability in their scores. Although intra-observer variability improved, inter-observer agreement remained a challenge, particularly for ballooning (Figure 4). Dr. Zabbatino suggested that pathologists might second-guess themselves in efforts to stay consistent with colleagues, a factor contributing to variability.
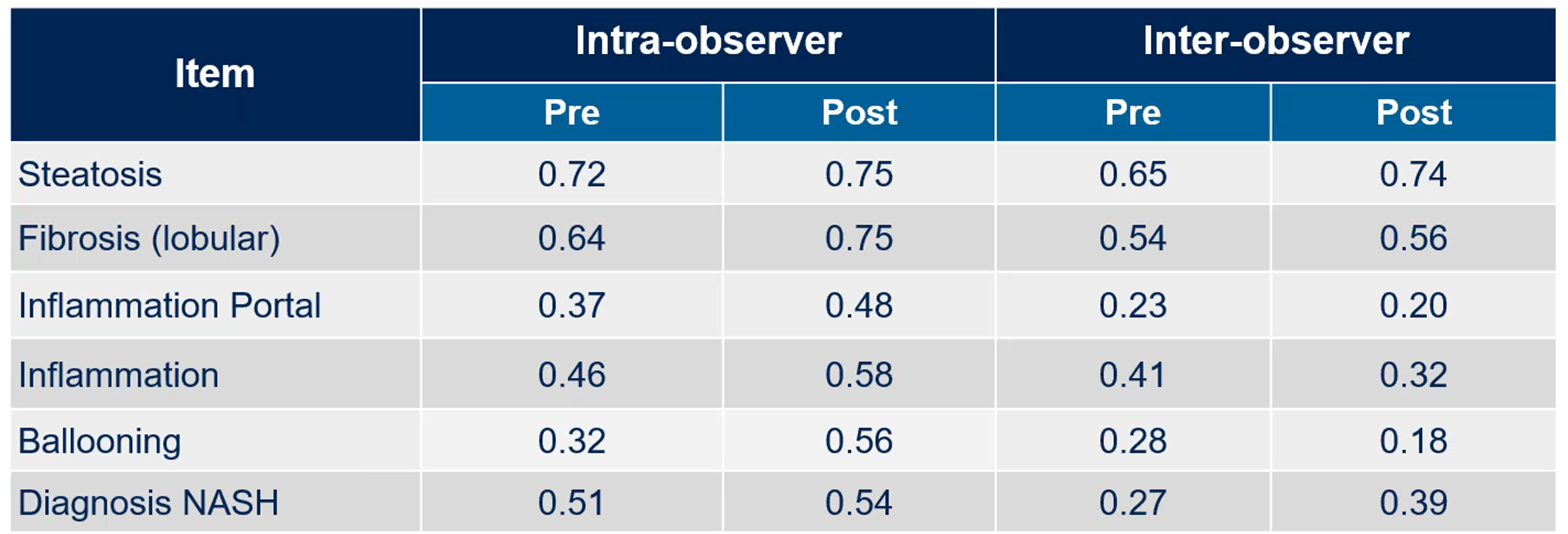
Figure 4. Impact of reader training: two pathologists, 65 liver biopsies. Adopted from Gawrieh et al. Effects of interventions on intra-and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagn Pathol. 2011 15(1): 19-24.
Therefore, achieving consistent NAS scoring remains challenging, even among experienced pathologists, due to significant variability in interpreting liver biopsy features like steatosis, inflammation and ballooning.
Dr. Zabbatino noted that refining MASH scoring methods and setting robust eligibility criteria can help consistently assess patient populations from trial start to endpoint. This would improve the ability to detect meaningful treatment effects over time.
Evolution of Pathology Read Paradigms in MASH Clinical Trials
Pathology reading methods in MASH trials have evolved to reduce variability and improve data reliability.
Dr. Zabbatino discussed how the field has moved from the use of single-reader glass slide reviews to sophisticated multi-reader digital slide reviews that enhance consistency across studies (Figure 5).
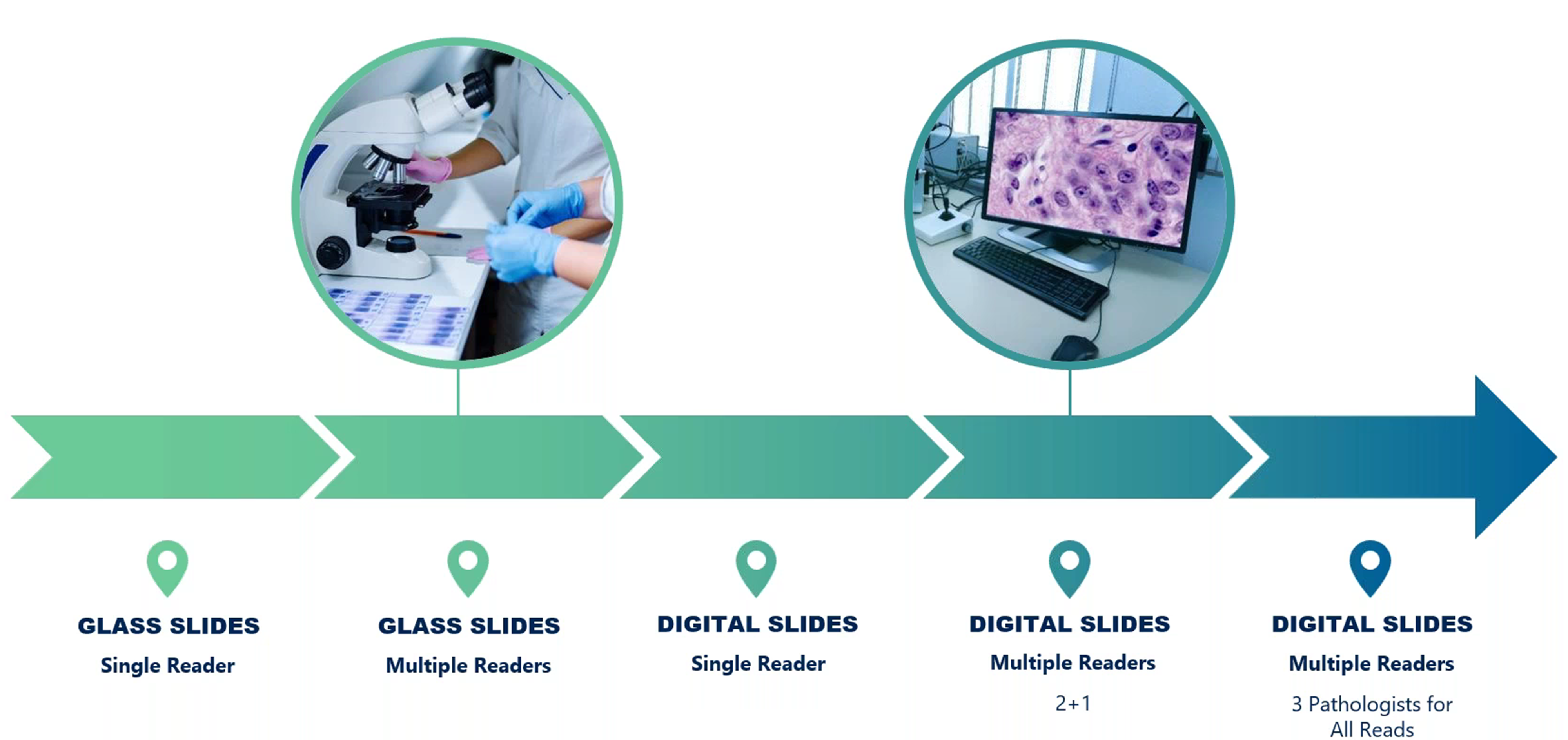
Figure 5. Evolution of pathology read to help minimize variability in final pathology scoring.
In early MASH studies, glass slides were often read by a single pathologist, creating logistical challenges and increased variability because of subjective interpretation. As Zabbatino explained, this prompted the transition to a multi-reader model to introduce consensus among pathologists.
However, the limitations of glass slides remained a concern, as shipping them between locations posed risks of breakage and extended turnaround times. The adoption of digital slides helped alleviate these issues, providing faster and more secure access for pathologists across multiple locations.
The initial digital slide model adopted the “2+1” approach, where two independent pathologists reviewed each slide, with an adjudicator resolving any discrepancies. However, study sponsors and key opinion leaders might have varying preferences, with some opting to go to an adjudicator only on areas of discordance, while others preferring a third, independent review. This sometimes resulted in three separate scores, making it challenging to achieve a true consensus on scoring.
To further refine this, a three-reader model was adopted, where three pathologists independently review each slide; the outcomes of this most recent central review paradigm are shown in Figure 6.
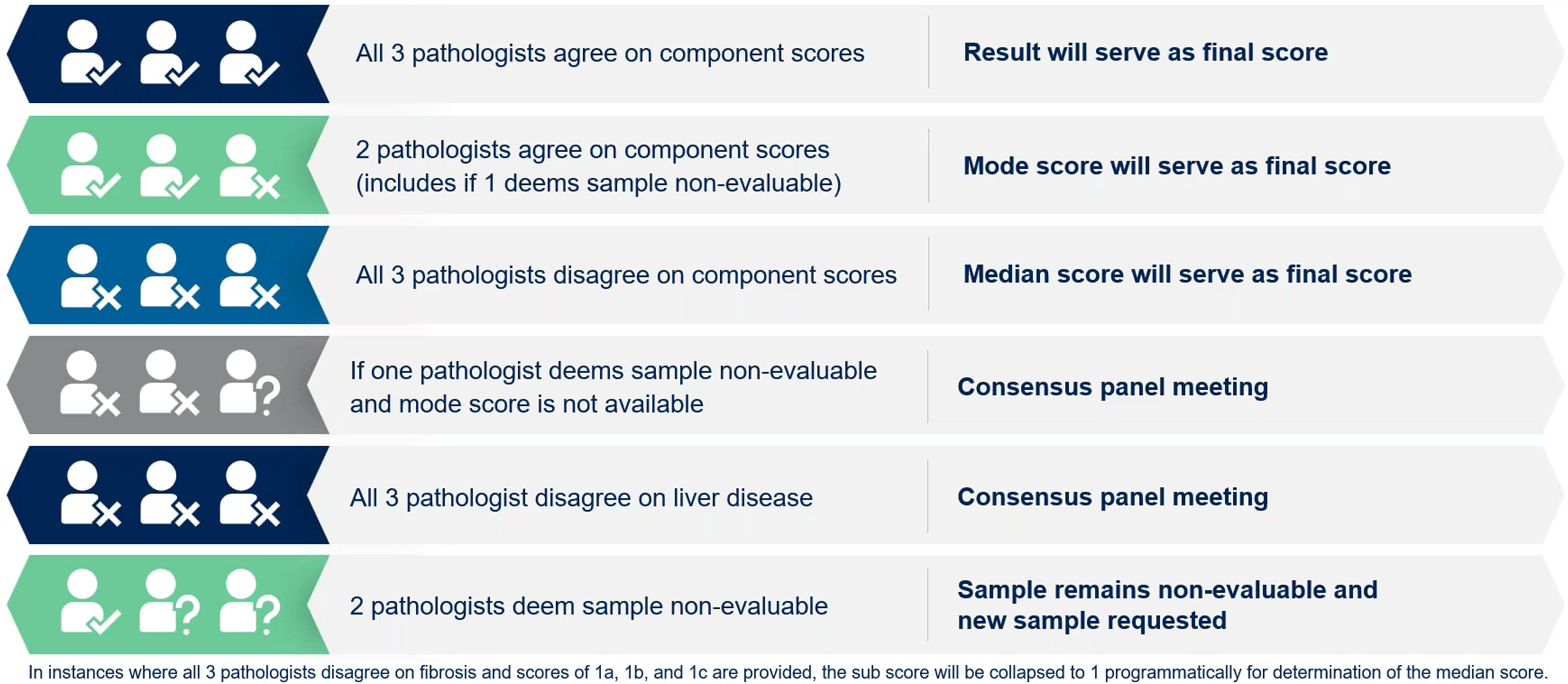
Figure 6. Most recent central review paradigm.
Slide preparation and staining variability presented another obstacle to consistent scoring. Dr. Zabbatino explained the benefits of processing standardized, unstained slides at a central lab, such as Medpace Reference Laboratories (MRL), which ensures uniform staining for all study samples. This practice minimizes discrepancies caused by different local staining techniques, which reduces variability in pathologist interpretations and supports reliable scoring outcomes.
“We use a set of sample slides that the pathologists will review and then we meet with them to ensure that they get an opportunity to discuss those cases where they’ve disagreed, so that hopefully during the course of the study, we can increase our concordance.”
— Salvatore Zabbatino
To help maintain consistency, Medpace integrates an initial review phase, where all participating pathologists assess a set of sample slides together. This collaborative step allows pathologists to discuss any disagreements, refining their approach to help ensure consistent scoring during the trial.
By using digital multi-reader platforms, standardizing staining processes and fostering concordance among pathologists, Medpace’s innovative pathology reading methods contribute to more robust data integrity.
The Future of MASH Clinical Trials
Liver biopsies remain a critical tool in MASH clinical research. While non-invasive diagnostic tests are advancing, they are not yet suitable replacements for biopsy in assessing MASH endpoints. The transition to digital histology and the adoption of a centralized multi-reader system has marked a substantial step forward, setting new standards for data accuracy and endpoint reliability.
As the field of MASH research progresses, continued innovation in trial design and methodology will be needed to improve data quality, reduce patient burden and ultimately, bring new therapies to market for this pressing health concern.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN