The FDA has approved Palsonify (paltusotine) for adults with acromegaly who did not respond well to surgery or cannot have surgery. Palsonify is now the first once-daily oral treatment approved for this condition.
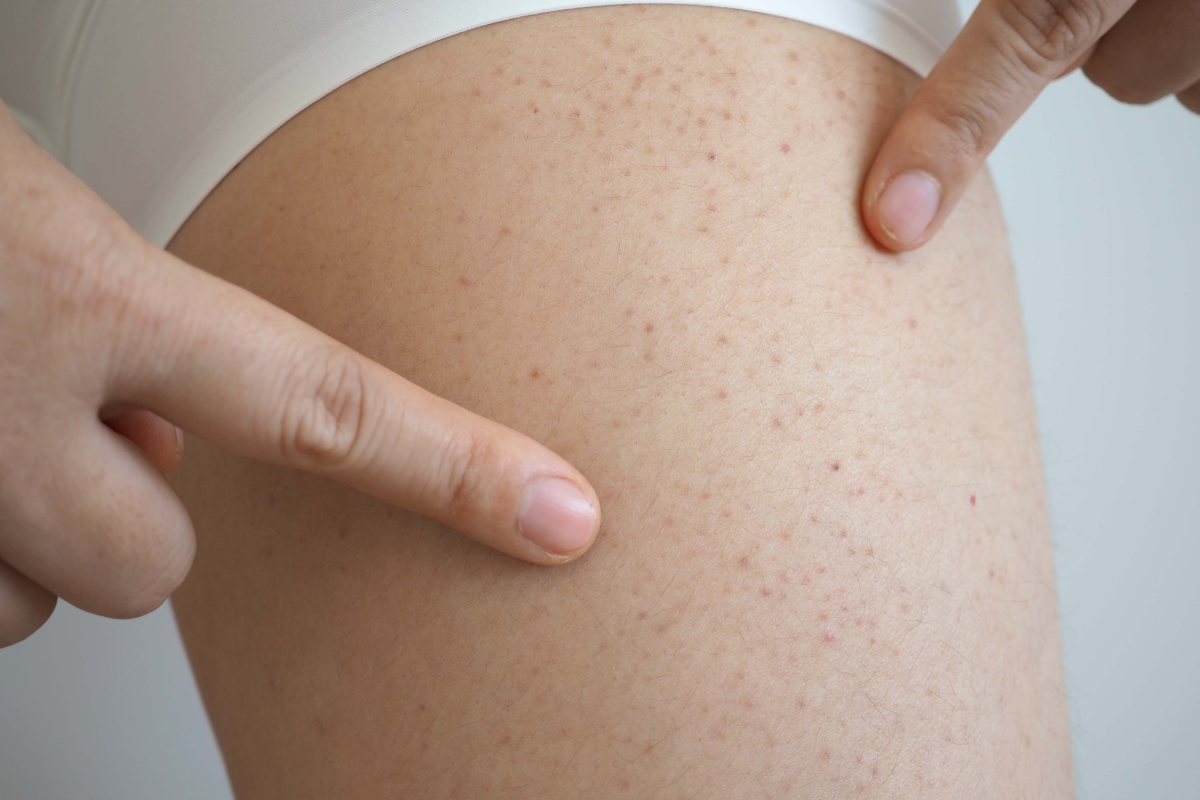
Acromegaly is a rare disease where a tumor in the pituitary gland makes too much growth hormone.
Palsonify is a daily pill that blocks those extra signals to bring hormone levels back to normal. Developed by Crinetics Pharmaceuticals, a San Diego company focused on endocrine diseases, this is the firm’s first marketed product and a milestone in its small-molecule portfolio.
What Is Acromegaly?
Acromegaly is caused by a benign pituitary tumor that leads to excess growth hormone and high levels of insulin-like growth factor 1 (IGF-1). This imbalance causes abnormal growth, changes in appearance and serious health problems.
Around 30,000 people in the US have the condition, but it is often missed or mistaken for other issues, and diagnosis is typically delayed by about eight years.
Without treatment, life expectancy can be shortened by up to 10 years due to complications such as diabetes and heart disease.
Surgery is usually the first approach, but long-term remission occurs in about half of patients. About two-thirds need ongoing medication, often injections, to keep hormone levels in check.
Standard drugs include somatostatin receptor ligands and pegvisomant (brand name Somavert), a growth hormone receptor blocker, but many people do not achieve full control of the disease.
XTALKS WEBINAR: Every Patient Matters: Bespoke Digital Endpoint Strategies for Rare Disease Drug Development
Live and On-Demand: Wednesday, November 12, 2025, at 11am EDT (5pm CET/EU-Central)
Register for this free webinar to learn how to select, operationalize and protect endpoints that stand up to regulatory scrutiny and support confident decision-making in rare disease drug development.
What Is Paltusotine and How Did it Perform in Clinical Trials?
Paltusotine is a somatostatin receptor type 2 (SST2) agonist. In simple terms, it mimics the body’s natural somatostatin hormone, which helps control growth hormone release. By blocking excess pituitary signals, the drug lowers growth hormone and IGF-1 levels.
Unlike older medications requiring injections, Palsonify is taken once daily by mouth, easing treatment.
The approval is based on two Phase III trials, PATHFNDR-1 and PATHFNDR-2. In a trial of 111 adults, 56% on paltusotine achieved normal hormone levels versus 5% on placebo after 24 weeks.
In the second trial, which included 58 adults already responding to medical therapy, 83% of those switched to paltusotine maintained hormone control at 36 weeks, compared with 4% on placebo. Participants also reported fewer symptoms, measured with the FDA-aligned Acromegaly Symptom Diary, which tracks headaches, sweating, fatigue, swelling and joint pain.
Long-term data from open-label extension (OLE) studies, shared at the Endocrine Society’s 2025 meeting, showed most patients continued treatment (91% from PATHFNDR-1 and 97% from PATHFNDR-2) with sustained hormone control and symptom relief.
Paltusotine was generally well tolerated. The most common side effects included digestive issues like diarrhea, nausea and abdominal pain, along with appetite changes and fluctuations in blood sugar or heart rate.
Less common but more serious risks include gallstones, thyroid or heart rhythm changes, and vitamin B12 deficiency, requiring regular monitoring.
Next Steps and Emerging Contenders
According to Crinetics, Palsonify will be available in the US in early October. The company has also sought approval in Europe, with a decision expected in 2026, and is collaborating with partners in Japan. The drug is also in Phase III testing for carcinoid syndrome, a complication of neuroendocrine tumors.
To support access, Crinetics has partnered with Orsini, a specialty pharmacy focused on rare diseases, to distribute the drug and offer patient support services.
The approval comes as other therapies are advancing globally. In August, the UK Medicines and Healthcare products Regulatory Agency (MHRA) approved Camurus’ Oczyesa (CAM2029), a once-monthly octreotide depot that can be self-administered with an autoinjector, providing another option to lessen treatment burden.
Pipeline development also continues, as Marea Therapeutics recently began a Phase I trial of MAR002, a long-acting growth hormone receptor antagonist antibody, reflecting early efforts to expand treatment options.
If you want your company to be featured on Xtalks.com, please email [email protected].









Join or login to leave a comment
JOIN LOGIN