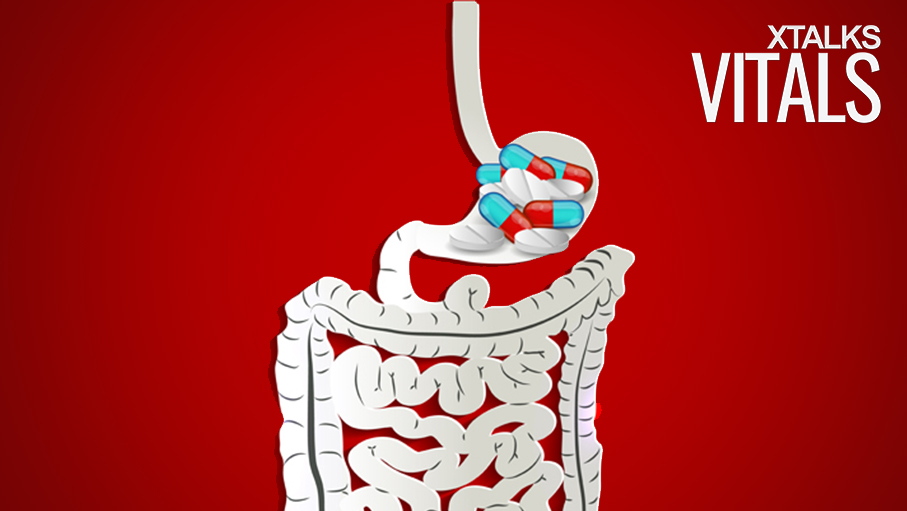
A new pill designed to adhere to the lining of the gastrointestinal tract and steadily release its drug load, has been developed by researchers at MIT and Brigham and Women’s Hospital. Once swallowed, one side of the tablet binds to the intestinal tissue, while the other resists passing food and liquids which could dislodge it from its point of attachment.
According to the researchers, this type of extended-release pill could help reduce the required dosage frequency for many medication. The technology could improve medication adherence for drugs like antibiotics that normally need to be taken two to three times per day.
“This could be adapted to many drugs. Any drug that is dosed frequently could be amenable to this kind of system,” said Giovanni Traverso, a research affiliate at MIT’s Koch Institute for Integrative Cancer Research, a gastroenterologist at Brigham and Women’s Hospital, and a senior author on the publication. The details of the device were published in the journal, Advanced Healthcare Materials.
Traverso and his colleagues in Robert Langer’s lab at the Koch Institute, have long been focused on the development of materials capable of adhering to skin – or being implanted into the body – to facilitate long-term drug release. To design a device capable of steadily releasing a drug into the gastrointestinal tract, the researchers used materials known as mucoadhesives, which are capable of sticking to the lining of the stomach or the gut.
While previous studies have tried to employ this technology to facilitate gastrointestinal drug delivery, materials flowing through the intestine have often broken the bond between the pill and lining before the entire drug load has been released. “The challenge with mucoadhesives is that the GI tract is a very rough and abrasive environment,” said Young-Ah Lucy Lee, a technical assistant at the Koch Institute.
Langer and his associates developed a double-sided device – known as a Janus device – to overcome the challenges associated with this type of drug delivery. One side of the pill is coated with a mucoadhesive, while the other contains an omniphobic compound, allowing it to repel any material it comes in contact with.
In order to test the ability of the Janus pill to stay in place, the researchers used pig intestines through which a liquid food mixture was passed to simulate the natural environment inside the GI tract. Compared to a dual-sided omniphobic tablet and a dual-sided mucoadhesive tablet, (which only lasted seconds in the simulated environment), the Janus pill remained attached to the lining for approximately 10 minutes – the duration of the experiment.
“The ability to precisely engineer the adhesiveness of a particle opens up possibilities of designing particles to selectively adhere to specific regions of the GI tract, which in turn can increase the local or systemic concentrations of a particular drug,” said Tejal Desai, a professor of bioengineering and therapeutic sciences at the University of California at San Francisco.
The researchers now plan to conduct further animal tests to determine how long the pills remain attached to the gut. In addition, they are interested in determining the rate at which drugs are released from the pill, and how the pill might be engineered to target specific areas within the GI tract.






Join or login to leave a comment
JOIN LOGIN