A 20-year-old patient with B-cell acute lymphoblastic leukemia (ALL) who relapsed nine months after receiving the CAR-T cell therapy developed by researchers at the University of Pennsylvania School of Medicine died as a result of a single genetically-engineered leukemic cell, according to a new study published in the journal Nature Medicine. The researchers believe that a single cancer cell was allowed to proliferate without being targeted by the CAR-T cell therapy due to the presence of a chimeric antigen receptor (CAR), which eventually led to the patient’s fatal relapse after months of remission.
“We learn so much from each patient, in both success or failure of this new therapy, that helps us improve these still-developing treatments so they can benefit more patients,” said senior study author Dr. J. Joseph Melenhorst, an associate professor of Pathology and Laboratory Medicine and a member of Penn’s Center for Cellular Immunotherapies. “This is a single case, but is still incredibly important and can help us refine the intricate processes required for manufacturing CAR-T cell therapy to ensure the best chance of long-term remissions.”
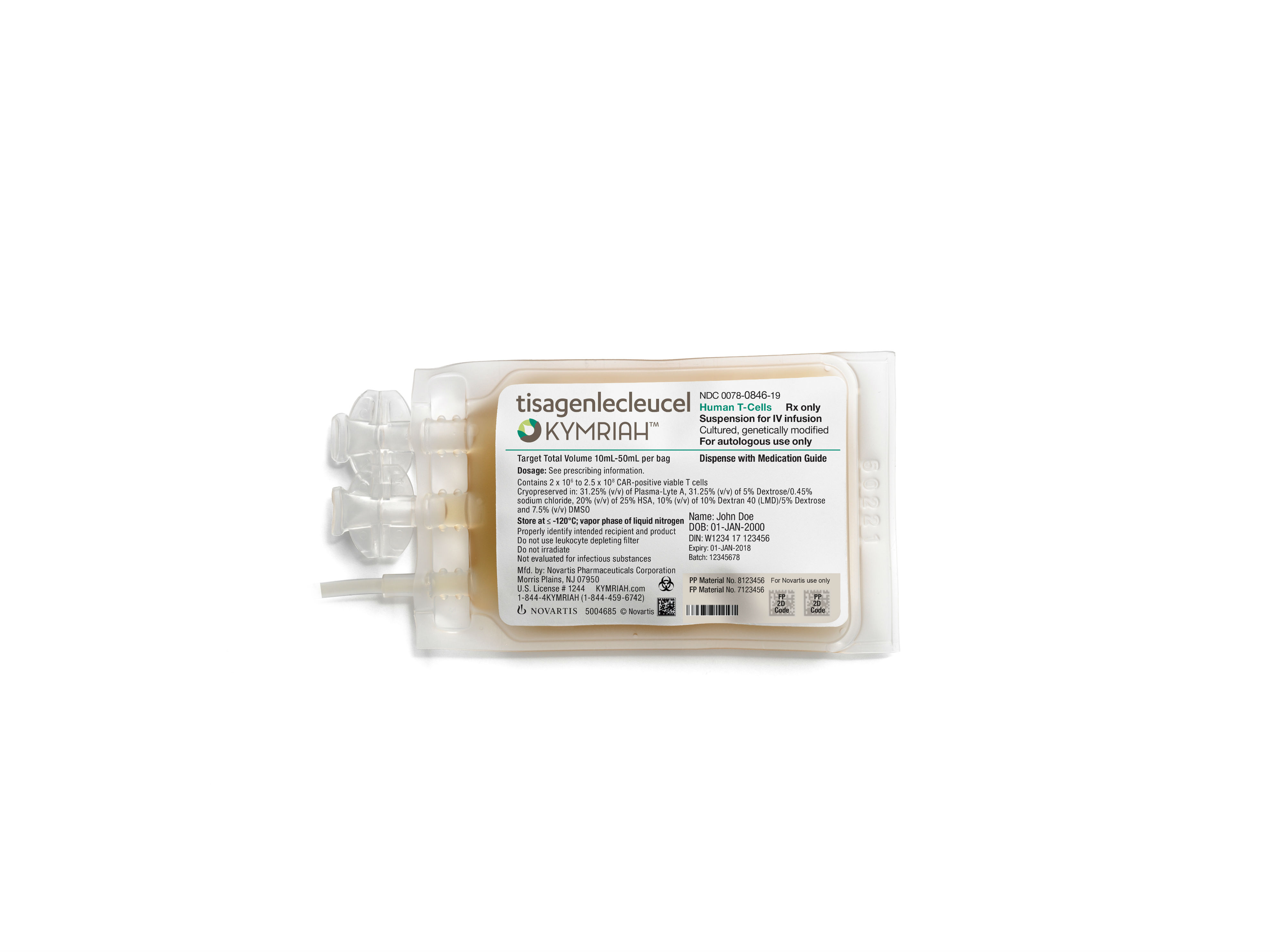
CAR-T cell therapies represent the cutting edge of oncology treatments. The therapy developed by researchers at Penn’s Perelman School of Medicine and at Children’s Hospital of Philadelphia (CHOP) – which is now known as Novartis’ Kymriah – engineers a patient’s own T cells with a CAR from a lentivirus which allows them to recognize and destroy any leukemic cells that express the CD19 marker.
The patient who is the focus of the current study had experienced three prior relapses before participating in the Phase I clinical trial of Kymriah – involving 369 patients total – in 2016. While the patient had nine months of remission after being reinfused with the modified immune cells, the eventual relapse proved to be fatal.
After relapse, the patient was tested to confirm that their leukemic cells actually expressed CD19, with clinicians finding that the marker was not detectable. While this occurrence is a not-uncommon cause of relapse – accounting for about 60 percent recurrences in patients with ALL – a much rarer event was found to be the cause the fatal return of cancer in this patient.
During engineering of the patient’s T cells with the CAR, a single leukemic cell was also edited to include the cancer-fighting CAR, allowing it to evade detection by the immune system. When the study investigators analyzed this patient’s leukemia cells after relapse, they found that they all expressed the CAR protein.
RELATED: Kymriah Manufacturing Issues Spell Trouble for Commercialization of Novartis CAR-T Therapy
“In this case, we found that 100 percent of relapsed leukemic cells carried the CAR that we use to genetically modify T cells,” said the study’s lead author Dr. Marco Ruella, an assistant professor of Hematology-Oncology at Penn. “This is the first time in hundreds of patients treated at Penn and other institutions that we’ve observed this mechanism of relapse, and it provides important evidence that due to the delicate and complex manufacturing process any slight variation can have an impact on patient outcomes.”
The findings of this single case study highlight the need for stringent manufacturing specifications for Kymriah and other CAR-T therapies to prevent future occurence of the type of cell contamination seen here. However, for those concerned that this same problem could occur in patients currently being treated with Kymriah – which was approved by the FDA about a year ago – Novartis has some reassuring words.
“The Novartis manufacturing process is different from the academic manufacturing process used at Penn,” a spokesperson for Novartis told BioPharma Dive. “We have processes in place to eliminate B-cells, including leukemic cells, from the apheresed product and have steps throughout the manufacturing process to further limit the risk of B-cell presence.”
Still, the Swiss drugmaker has faced some manufacturing challenges in the production of Kymriah for the treatment of diffuse large B-cell lymphoma (DLBCL). In July, Novartis announced it had been seeing some variability in batches of Kymriah. The news came not long after the company said they’d be partnering with Cell for Cure, a French CDMO, in an effort to set up manufacturing sites to serve European patients.
For now, it seems that issues surrounding the manufacturing of CAR-T cell therapies like Kymriah can be attributed to its status as a very new and personalized form of cancer treatment.
“It’s another demonstration of the unique (and often unforeseen) challenges of cell therapies, especially autologous therapies derived from cancer patients,” said Michael Gladstone, a principal at Atlas Venture. “However, it sounds like this has already been addressed by Novartis’s manufacturing improvements. This reflects the rapid pace of engineering progress in this arena.”









Join or login to leave a comment
JOIN LOGIN