Roche’s Ocrevus (ocrelizumab) has represented a valuable treatment option for patients with relapsing and progressive forms of multiple sclerosis (MS) since its approval in 2017, however new research presented at the 2018 American Academy of Neurology (AAN) Annual Meeting in Los Angeles has found that the drug may limit a patient’s ability to respond to routine vaccinations. The two studies conducted by neurologists at Penn Medicine provide important information on the effectiveness of vaccines in this patient population.
About 400,000 individuals in the US have been diagnosed with MS and it’s estimated that 10,000 new cases are identified each year. The genetic disease is characterized by progressive muscle weakness and pain which are caused by the demyelination of nerve cells.
Because MS is an autoimmune disease, Ocrevus was designed to target and destroy B cells displaying the CD20 protein on their cell surface which can become over-activated and exacerbate MS flare-ups. MS is also affected by interactions between other immune cells such as T cells, myeloid cells and B cells, and Ocrevus has been shown to limit B cell activation in patients’ blood.
The first study looked at the immune cell markers in MS patients before and after they received treatment with Ocrevus. They found that at 12 and 24 weeks after treatment, inflammation and injury markers in the spinal fluid consistent with MS pathology were reduced. Their findings also highlighted the role of B cell and T cell interactions in mediating neuronal damage in MS patients.
RELATED: Roche’s Multiple Sclerosis Drug is First Disease-Modifying Therapy Approved by FDA
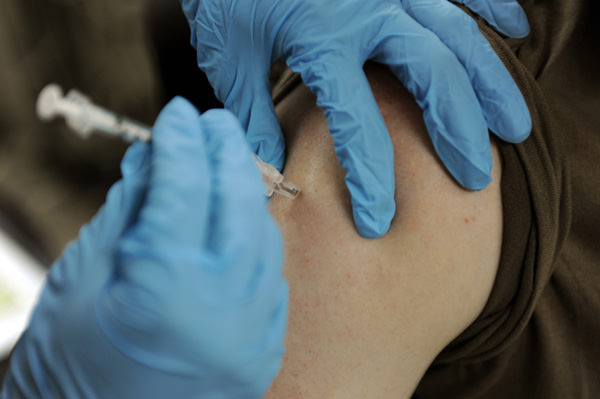
The second study presented at the AAN annual meeting compared the vaccine responses to tetanus, flu and pneumococcus in patients who were treated with Ocrevus (treatment group) and those who were not (control group). While all the patients successfully mounted a response against the antigens in the vaccines, the strength of the response was generally lower among MS patients being treated with Ocrevus.
While 55 percent of patients not given Ocrevus showed an immune response to the tetanus vaccine at eight weeks, just 24 percent of those given the MS drug showed the same response to vaccination. An attenuated response was also observed for the other vaccines, with 100 percent of the control group and 72 percent of the treatment group showing a response to the pneumococcal vaccine, and up to 97 percent of the control group and 80 percent of the treatment group mounting immunity to five influenza strains.
“This study shows that while people with MS treated with ocrelizumab can still mount vaccine responses, it’s not nearly as strong as prior to treatment,” said neurologist Dr. Amit Bar-Or, chief of the Multiple Sclerosis division and director for the Center for Neuroinflammation and Experimental Therapeutics at Penn Medicine. “While antibody responses were reduced in the ocrelizumab treated patients, they still responded to a certain level. This is valuable information in terms of seasonal vaccines such as the flu – it appears safe for patients taking ocrelizumab to get vaccinated and vaccination is likely to provide them with at least some protection from such infections.”
According to Bar-Or and his colleagues, the findings emphasize the current clinical guidance for prescribing Ocrevus which maintains that patients should receive the necessary vaccinations before starting treatment, preferably six weeks prior. Ocrevus was developed by Roche subsidiary Genentech, which sponsored the studies.
“Translational research like this work with ocrelizumab is an example of what we’re trying to do at Penn,” said Bar-Or, who was also senior author on the study published in the journal Neurology. “Research like this allows us to learn more both about the mechanisms underlying MS activity and injury, as well as the biology of MS treatments, which in turn will help us better individualize treatments for specific patients.”








Join or login to leave a comment
JOIN LOGIN