The US Food and Drug Administration (FDA) has accepted KalVista Pharmaceuticals’ New Drug Application (NDA) for sebetralstat, a novel, investigational oral plasma kallikrein inhibitor for the on-demand treatment of hereditary angioedema (HAE) attacks in adult and pediatric patients aged 12 years and older.
If approved, sebetralstat would be the first oral, on-demand treatment for HAE. The FDA has set a Prescription Drug User Fee Act (PDUFA) date of June 17, 2025, by which it will make its decision on the treatment. The agency does not plan to seek input on the application from an advisory committee.
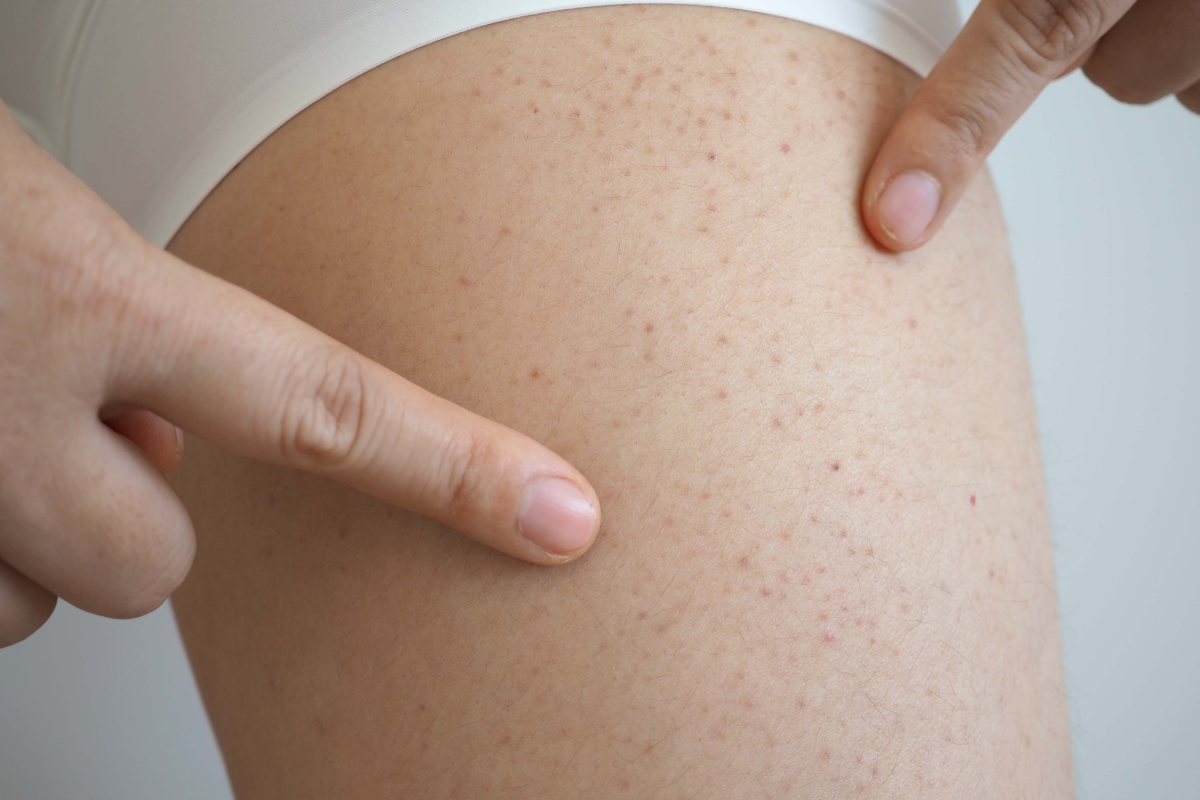
HAE is a rare genetic condition that causes episodes of severe swelling in various parts of the body, including the face, hands, feet and airway. These attacks can be unpredictable, often leading to life-threatening situations when they affect the throat or lungs. HAE is caused by a deficiency or dysfunction of the C1-inhibitor, a protein involved in regulating inflammation.
HAE affects approximately one in 50,000 people globally, and there is currently no cure for the disease.
Currently available treatments focus on managing symptoms and preventing attacks. This includes C1-inhibitor (C1-INH) replacement therapy, which involves intravenous C1-INH concentrates (e.g., Cinryze, Haegarda) used to replace the deficient or dysfunctional C1 inhibitor protein in the blood, helping to prevent the inflammatory processes that cause angioedema. Subcutaneous C1-INH is an alternative for patients preferring or needing at-home administration.
XTALKS WEBINAR: Enhancing Lab Operations: Best Practices for Risk Mitigation and Preparedness Planning
Live and On-Demand: Wednesday, October 16, 2024, at 1pm EDT (10am PDT)
Register for this free webinar to learn about risk mitigation and preparedness planning in laboratory operations and how they help maintain laboratory standards, manage staff and ensure biosecurity.
Sebetralstat works by inhibiting plasma kallikrein, an enzyme that plays a crucial role in the inflammatory processes that lead to angioedema attacks. Unlike existing treatments that may require complex preparation and delivery, sebetralstat’s oral mode of administration offers convenience and faster access to treatment, which is critical for patients who may need immediate intervention.
“We are thrilled with the FDA’s acceptance of our NDA for sebetralstat as it moves us one step closer to bringing a potentially transformative therapy to the HAE community,” said Ben Palleiko, chief executive officer at KalVista in a press release.
“The compelling data included in our NDA package show that sebetralstat has the potential to significantly alter the way people treat and manage their disease,” he said.
He also noted the positive reception and support for sebetralstat: “Given that it could be the first, oral on-demand treatment for HAE, we continue to receive strong support and hear a sense of urgency among healthcare providers, advocates, patients and their families for sebetralstat.”
Related: FDA’s New Rare Disease Innovation Hub to Elevate Patient Care
The NDA submission was supported by data from the KONFIDENT Phase III clinical trial and ongoing KONFIDENT-S open-label extension trial.
In the trials, sebetralstat demonstrated a significant reduction in attack severity and duration compared to placebo. The treatment was well-tolerated, with a safety profile similar to placebo.
Both the 300 mg and 600 mg formulations of sebetralstat achieved the beginning of symptom relief significantly faster than placebo.
In KONFIDENT-S, sebetralstat enabled patients to treat attacks early with a median time of nine minutes from attack onset to treatment. It also included a median time of 1.3 hours to the beginning of symptom relief for laryngeal attacks. The drug demonstrated a consistent safety and efficacy profile with KONFIDENT.
KalVista will present further data from the KONFIDENT and KONFIDENT-S trials at the 2024 Annual Scientific Conference of the American College of Allergy, Asthma and Immunology Conference in Boston from October 24 to 28.
Along with the NDA acceptance, KalVista recently announced that the European Medicines Agency (EMA) validated the company’s Marketing Authorization Application (MAA) for sebetralstat. KalVista said it expects to file for approval in the UK, Japan and other countries later in 2024.
If you want your company to be featured on Xtalks.com, please email [email protected].









Join or login to leave a comment
JOIN LOGIN