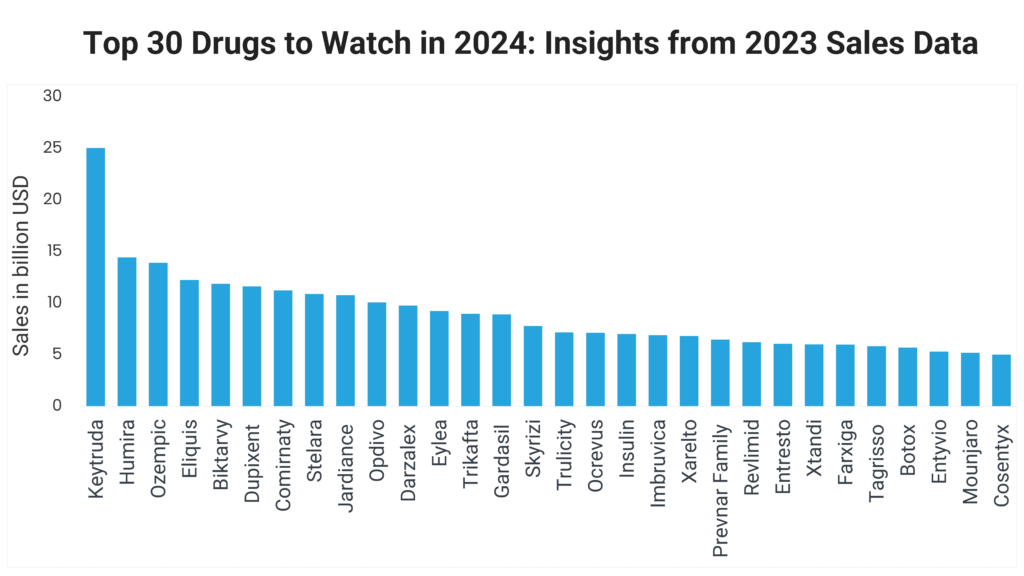
The pharma industry is poised for significant transformations in 2024, driven by groundbreaking drugs that have set new benchmarks in the industry. This blog delves into the top 30 drugs to watch in 2024, leveraging insights from their sales performance in 2023. These medications have not only redefined therapeutic standards across various medical conditions but have also showcased impressive sales figures, innovative advancements and expanded indications.
In this detailed exploration, we begin with a closer look at the best-selling drugs from last year, such as Merck’s oncology powerhouse Keytruda (pembrolizumab), which topped the charts with sales of $25.011 billion, and AbbVie’s Humira (adalimumab), a long-standing market leader in immunology that faced its first major biosimilar competition. We will also examine the rising stars like Novo Nordisk’s Ozempic (semaglutide), which has become a frontrunner in diabetes management, and the dynamic performance of Gilead Sciences’ Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide) in the HIV treatment landscape.
Join us as we uncover the stories behind these pharmaceutical giants, the strategic maneuvers of their developers and the future prospects that make them the drugs to watch in 2024. This journey through the pharmaceutical market will provide valuable insights into the trends shaping the healthcare industry and the innovations that continue to push the boundaries of medical science.
Note: When it comes to companies that report in foreign currencies, the conversion to US dollars uses the average annual exchange rates reported by the US Federal Reserve.
1. Keytruda (Pembrolizumab)
Keytruda 2023 Sales: $25.011 billion
Company/Developer: Merck
Date of First US Food and Drug Administration (FDA) Approval: September 4, 2014
Indications Keytruda Is FDA-Approved for: Unresectable or metastatic melanoma. Keytruda is specifically indicated for the adjuvant treatment of adult and pediatric (12 years and older) patients with stage IIB, IIC or III melanoma following complete resection. It is also approved to treat non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma (PMBCL), urothelial carcinoma, microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) cancer, MSI-H or dMMR colorectal cancer, gastric cancer, esophageal cancer, cervical cancer, hepatocellular carcinoma, biliary tract cancer, Merkel cell carcinoma, renal cell carcinoma, endometrial carcinoma, tumor mutational burden-high (TMB-H) cancer, cutaneous squamous cell carcinoma (cSCC) and triple-negative breast cancer.
List Price of Keytruda: According to Keytruda’s website, the list price for each indicated dose of Keytruda when given every three weeks is $11,337.36 and when given every six weeks is $22,674.72. The indicated adult dose of Keytruda is either 200 mg administered every three weeks or 400 mg every six weeks.
Why It Sold So Well: In 2023, Keytruda finally topped the list as the world’s best-selling drug. The first-place crown was expected after AbbVie’s Humira (adalimumab) reached its patent cliff and a slew of biosimilars began to hit the market. Keytruda was already a best-seller among oncology drugs and in 2023, sales grew 21 percent at constant exchange rates to $25 billion.
Keytruda also won just under half a dozen FDA approvals in new oncology indications. The most notable of these include use as a neoadjuvant-adjuvant therapy around surgery in resectable NSCLC, which makes it the first PD-1/L1 inhibitor regimen approved for use both before and after surgery; approval as a first-line treatment in combination with Astellas and Pfizer’s Padcev (enfortumab vedotin) for locally advanced or metastatic urothelial cancer; and as first-line treatment in combination with chemotherapy for locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma.
Keytruda’s reign as the world’s number one selling drug may be short, though, given the massive popularity of glucagon-like peptide-1 (GLP-1) agonists for type 2 diabetes and obesity treatment. In 2023, sales of Novo Nordisk’s semaglutide products Ozempic, Wegovy and Rybelsus totaled 145.8 billion DKK ($21.1 billion USD). Meanwhile, Eli Lilly’s dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 agonist Mounjaro (tirzepatide) amassed $5.2 billion in 2023 and its obesity counterpart Zepbound (tirzepatide) brought in more than $175 million in its first quarter on the market.
Keytruda will have a few more years to enjoy its strong market run as the drug is slated to lose patent exclusivity in 2028.
2. Humira (Adalimumab)
Humira 2023 Sales: $14.404 billion
Company/Developer: AbbVie
Date of First FDA Approval: December 31, 2002
Indications Humira Is FDA-Approved for: Rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa and uveitis
Cost of Humira: The price of two Humira subcutaneous kits (40 mg/0.4 mL each) is around $7,299.
Why It Sold So Well: Humira was finally knocked from the top position as the world’s best-selling drug. It enjoyed the title for nine years, and despite hitting its patent expiration back in 2016, AbbVie went to great lengths to block biosimilar competition. This included building a controversial “patent thicket” around the drug by creating slews of dubious patents to create a market monopoly and seal out biosimilar competitors.
But Humira’s run has finally come to an end as sales of the drug dropped 32 percent in 2023 from the $21.2 billion it earned in 2022. The biosimilar erosion hit especially hard in the last quarter of the year with sales falling almost 41 percent to $3.3 billion globally.
Amgen rolled out its Humira biosimilar Amjevita early in 2023. Owing to a 2017 settlement with AbbVie, Amjevita had several months of exclusivity on the US market.
However, AbbVie isn’t washing its hands of Humira just yet. The company’s CEO Richard Gonzalez said at the 2023 J.P. Morgan Healthcare Conference that AbbVie was ready to fight the competition and had secured broad 2023 formulary access for Humira with “all of the major” payers.
Nevertheless, AbbVie is shifting its focus to its newer immunology players, Rinvoq (upadacitinib) and Skyrizi (risankizumab), to compensate for Humira. Earlier this year, company heads said they expect the drugs to bring in $16 billion in 2024 and more than $27 billion by 2027.
3. Ozempic (Semaglutide)
Ozempic 2023 Sales: 95.718 billion DKK ($13.892 billion USD)
Company/Developer: Novo Nordisk
Date of First FDA Approval: December 5, 2017
Indications Ozempic Is FDA-Approved for: Type 2 diabetes; reducing the risk of cardiovascular events such as heart attack, stroke or death in patients with diabetes and known heart disease
List Price of Ozempic: Ozempic 0.25 or 0.5 mg (1 x 1.5-mL pen) is $968.52; Ozempic 1 mg (1 x 3-mL pen) is $968.52; Ozempic 2 mg (1 x 3-mL pen) is $968.52.
Why It Sold So Well: Ozempic has taken the world by storm and there’s no looking back as it’s officially become the world’s top-selling diabetes drug and is also on pace to become the world’s best-selling drug.
Ozempic sales grew at a whopping 66 percent in constant currency year-over-year. Demand for GLP-1 agonist drugs is only on the rise, with talk all across Hollywood, from the Oscars to Saturday Night Live, and personal backing by celebrities like Oprah recently.
Although indicated for type 2 diabetes, the drug’s weight loss side effect made it the newest weight loss fad of sorts. Since 2022, Novo has been struggling with shortages of the drug due to the massive demand and supply chain constraints. Novo has also been contending with dupes of its semaglutide products, including obesity med Wegovy.
4. Eliquis (Apixaban)
Eliquis 2023 Sales: $12.206 billion
Company/Developer: Bristol Myers Squibb (BMS), Pfizer
Date of First FDA Approval: December 28, 2012
Indications Eliquis Is FDA-Approved for: Stroke prevention in atrial fibrillation; venous thromboembolism prevention; orthopedic surgery; venous thromboembolism treatment
List Price of Eliquis: A 30-day supply (60 tablets) of Eliquis (5 mg) is $594.10.
Why It Sold So Well: Sales of BMS’s billion-dollar blockbuster Eliquis were up four percent year-over-year. The Pfizer-partnered blood thinner remains the top oral anticoagulant worldwide. On a fourth-quarter earnings call earlier this year, BMS’s chief financial officer David Elkins said the company continues to expect “strong growth” in the US this year. Pfizer earned $6.747 billion from Eliquis in 2023, also a four percent increase. In some smaller countries, Pfizer holds full commercial rights for the drug.
Eliquis has been leading the factor Xa inhibitor market over Bayer and Johnson & Johnson’s Xarelto (rivaroxaban). Eliquis’ success owes to gains in the non-valvular atrial fibrillation market and demonstrated superiority over the traditional anticoagulant warfarin, which includes a lower risk of major bleeding and does not need regular monitoring like warfarin.
Eliquis’s patent is set to expire in 2026 after the US Patent and Trademark Office granted Eliquis a patent extension from February 2023 to November 2026. The FDA approved generics from Micro Labs Limited and Mylan Pharmaceuticals Inc. (now Viatris) back in 2019. Pfizer and BMS reached legal settlements with both companies while continuing to fight other generic makers, pushing the market launches of their generics to April 1, 2028. In Canada and the UK, generic competition has already begun, resulting in a ten percent decline in non-US markets last year.
Eliquis was also chosen last year as one of the first ten drugs that will be up for Medicare price negotiations beginning in 2026 under the Inflation Reduction Act (IRA). The $7,100 annual price tag of the drug in the US was also blasted by US Senator Bernie Sanders in a Health, Education, Labor and Pensions (HELP) committee hearing in February this year. At the hearing, he asked BMS CEO Chris Boerner why Eliquis retails for $900 annually in Canada and costs just $18 to manufacture.
5. Biktarvy (Bictegravir/Emtricitabine/Tenofovir Alafenamide)
Biktarvy 2023 Sales: $11.850 billion
Company/Developer: Gilead Sciences
Date of First FDA Approval: February 7, 2018
Indications Biktarvy Is FDA-Approved for: HIV-1
List Price of Biktarvy: $3,981 per month
Why It Sold So Well: Biktarvy’s $11.85 billion in sales accounted for almost 44 percent of Gilead’s overall product sales last year and more than half of the company’s HIV portfolio, which is its biggest sales driver. According to Gilead’s 2023 annual report, sales of Biktarvy increased by 14 percent year-over-year in 2023 due to higher demand and also “higher average realized price.”
Biktarvy sales increased by seven percent to $3.1 billion in the fourth quarter of 2023 compared to the same period in 2022, which the company said reflects “higher demand, partially offset by lower average realized price due to channel mix.”
Gilead is expecting its HIV franchise, spearheaded by Biktarvy, to grow by four percent this year due to yearly increases in demand and Biktarvy’s growing market share.
6. Dupixent (Dupilumab)
Dupixent 2023 Sales: $11.588 billion
Company/Developer: Regeneron and Sanofi
Date of First FDA Approval: March 28, 2017
Indications Dupixent Is FDA-Approved for: Adults and children six months of age and older with moderate-to-severe eczema (atopic dermatitis or AD); maintenance treatment of moderate-to-severe eosinophilic or oral steroid-dependent asthma in adults and children six years of age and older whose asthma is not controlled with their current asthma medicines; with other medicines for the maintenance treatment of chronic rhinosinusitis with nasal polyposis (CRSwNP) in adults whose disease is not controlled; adults and children one year of age and older with eosinophilic esophagitis (EoE); adults with prurigo nodularis (PN)
List Price of Dupixent: $3,803.20 per carton
Why It Sold So Well: Dupixent had another strong year of growth in 2023 with net global sales increasing 33 percent to $11.588 billion in 2023 compared to 2022. Fourth quarter 2023 sales increased 31 percent to $3.22 billion versus fourth quarter 2022.
Since its FDA approval in 2017, Regeneron and Sanofi’s Dupixent was touted to be a major breakthrough for patients with AD, with industry analysts dubbing it to be “life changing.”
Since then, Regeneron has secured approvals in additional indications, most notably asthma. Expansions into children for both asthma and EoE further boosted its appeal, leading to huge leaps in sales every year, from $2.3 billion in 2019 to $4 billion in 2020 to $6.2 billion in 2021 and $9.8 billion in 2022.
The drug looks to continue its upward trajectory as Regeneron and Sanofi are looking to secure approval in chronic obstructive pulmonary disease (COPD), which would make it the first biologic approved for COPD. According to Schimmer, the approval would add $2.5 billion in peak sales for Regeneron in the US and another $1 billion for Sanofi, which handles Dupixent in other countries.
7. Comirnaty (COVID-19 Vaccine, mRNA)
Comirnaty 2023 Sales: $11.220 billion
Company/Developer: Pfizer and BioNTech
Date of First FDA Approval: August 23, 2021
Indications Comirnaty Is FDA-Approved for: Prevention of COVID-19
Cost of Comirnaty: Comirnaty intramuscular suspension (30 mcg/0.3 mL) costs about $10 for a supply of 18 mL.
Why It Sold So Well: Pfizer’s COVID-19 vaccine was expected to have an unprecedented run that would be short-lived as a hopeful pandemic-ending tool. In its first full year on the market in 2021, it earned $36.8 billion, the highest revenue generated in a single year for a single pharmaceutical product. The following year, sales inched upwards to $37.8 billion, surpassing fellow COVID-19 vaccine rival Spikevax from Moderna, which amassed $36 billion in two years.
Because of Comirnaty, Pfizer hit a record-breaking $100.33 billion in sales in 2022, the most revenue generated by any pharma company in a single year. As the pandemic cooled off in 2023, sales of Pfizer and BioNTech’s jointly developed shot fell by 70 percent to just over $11 billion. Pfizer’s total sales plummeted by more than 40 percent to $58.5 billion in 2023 due to declining sales of both its vaccine and COVID-19 antiviral Paxlovid (nirmatrelvir/ritonavir).
8. Stelara (Ustekinumab)
Stelara 2023 Sales: $10.858 billion
Company/Developer: Johnson & Johnson
Date of First FDA Approval: September 25, 2009
Indications Stelara Is FDA-Approved for: Plaque psoriasis, psoriatic arthritis, Crohn’s disease and ulcerative colitis
List Price of Stelara: $25,497.12 every eight weeks for the 90 mg dose
Why It Sold So Well: Stelara had another strong year and accounted for approximately 12.8 percent of J&J’s total revenues for 2023. In its 2023 annual report, the company said Stelara remains its largest product. It’s also bracing itself for biosimilar competition as Stelara reached its patent expiration last year. However, through settlements with biosimilar makers, the first ustekinumab biosimilars are not expected to launch until January 1, 2025. This includes Amgen’s biosimilar that was approved with an interchangeability tag under the brand name Wezlana in November. Following Wezlana’s launch will be biosimilars from Teva, Sandoz, Celltrion and Fresenius Kabi, which are also expected to hit the market in 2025.
J&J isn’t too worried about its blockbuster immunology drug succumbing to biosimilar erosion as it has a portfolio of 25 new potential runaway successes that will make up for Stelara. Biosimilars have already hit the European market, which the company expects will eat into sales in the second half of 2024.
9. Jardiance (Empagliflozin)
Jardiance 2023 Sales: $10.730 billion
Company/Developer: Boehringer Ingelheim and Eli Lilly
Date of First FDA Approval: August 1, 2014
Indications Jardiance Is FDA-Approved for: Type 2 diabetes, chronic heart failure, chronic kidney disease
List Price of Jardiance: $611.10 for a one-month supply
Why It Sold So Well: Jardiance was Boehringer Ingelheim’s top seller in 2023, accounting for 28.8 percent of the company’s total net sales last year. Sales of the drug were up 26.6 percent over 2022’s 5.832 billion euros ($6.143 billion USD).
For partner Eli Lilly, Jardiance was the company’s fifth best-seller in 2023, with $2.745 billion in sales, an increase of 33 percent compared to the $2.066 billion it earned in 2022.
In its 2023 annual report, Boehringer said the drug’s strong showing in 2023 reflected its continued “strong momentum” driven by new approvals in chronic kidney disease in Europe and the US last year, and a pediatric type 2 diabetes indication. Lilly also said the impressive sales growth was due to increased demand for the drug in the US and increased volume, along with some favorable impact of foreign exchange rates outside the US.
10. Opdivo (Nivolumab)
Opdivo 2023 Sales: $10.045 billion
Company/Developer: BMS and Ono Pharmaceutical
Date of First FDA Approval: December 22, 2014
Indications Opdivo Is FDA-Approved for: Melanoma, metastatic NSCLC, renal cell carcinoma, Hodgkin’s lymphoma, head and neck cancer, urothelial carcinoma, colorectal cancer, hepatocellular carcinoma, esophageal carcinoma, malignant pleural mesothelioma, gastric cancer
List Price of Opdivo: For Opdivo 240 mg every two weeks, the list price is $7,485 per infusion
Why It Sold So Well: Last year, Opdivo earned $10 billion in global sales, split at $9.009 billion for BMS and 145.5 billion Japanese yen (about $1.036 billion USD) for Ono Pharmaceutical. The PD-1 inhibitor was BMS’s second-largest revenue generator that saw continued growth in 2023. However, it hasn’t been able to keep pace with Merck rival Keytruda, which became the world’s top-selling drug in 2023. While Keytruda has racked up expanded approvals, Opdivo has been more modest in expansion. In March this year, Opdivo received FDA approval for use with chemo for the first-line treatment of bladder cancer. Keytruda got the same combination approval with Padcev (enfortumab vedotin) in the same indication.
Although Opdivo is set to go off patent in 2028, BMS is currently exploring it in Phase III trials for indications including muscle invasive urothelial carcinoma and hepatocellular carcinoma (HCC) as well as periadjuvant NSCLC.
BMS is also developing a subcutaneous version of the PD-1 inhibitor as is Merck, but BMS may score an earlier approval as it is further ahead in development. An Opdivo injectable could become the first PD-1/L1 inhibitor that is administered subcutaneously in the US. BMS said it aims to convert about 30 percent to 40 percent of the overall Opdivo US market to the subcutaneous formulation.
11. Darzalex (Daratumumab)
Darzalex 2023 Sales: $9.744 billion
Company/Developer: Janssen Biotech, Inc. (part of Johnson & Johnson) in collaboration with Genmab
Date of First FDA Approval: November 16, 2015, for Darzalex; May 1, 2020, for Darzalex Faspro
Indications Darzalex/Darzalex Faspro Are FDA-approved for:
- Darzalex: As an intravenous injection into the vein for multiple myeloma treatment in various stages (newly diagnosed and those who have received one or more prior lines of therapy), for patients both eligible and ineligible for autologous stem cell transplant and as a monotherapy in pre-treated patients.
- Darzalex Faspro: As a subcutaneous injection in the abdomen for multiple myeloma across similar settings as Darzalex, including for both autologous stem cell transplant-eligible and transplant-ineligible patients.
Price of Darzalex and Darzalex Faspro:
- Darzalex: Intravenous solution 20 mg/mL, from $758.34 for 5 mL
- Darzalex Faspro: Subcutaneous solution 1,800 mg-30,000 units/15 mL from $10,216.30 for 15 mL
Why They Sold So Well: Multiple myeloma is an incurable and the second most common form of blood cancer, with treatments focused on prolonging and improving the quality of life. In 2024, it’s estimated that more than 35,000 people will be diagnosed in the US alone.
Darzalex and Darzalex Faspro have revolutionized the treatment of multiple myeloma due to their innovative mechanism of action targeting the CD38 molecule, which is highly expressed on the surface of myeloma cells. This targeting helps in the direct killing of these cancerous cells and modulation of the immune system to enhance anti-tumor activity.
Darzalex Faspro offers the added advantage of subcutaneous administration, which is generally quicker and may be less painful than intravenous routes, improving patient compliance and comfort. These benefits have been clearly reflected in their rapid adoption in clinical practice and are backed by strong data from clinical trials showcasing their efficacy and safety. More recently, Darzalex-based regimens have shown profound clinical outcomes (such as improved progression-free survival [PFS] and high survival rates) in the PERSEUS, MAIA and CASSIOPEIA pivotal studies.
Genmab announced that for the first quarter of 2024, worldwide net sales of Darzalex and Darzalex Faspro amounted to $2.692 billion. The US accounted for $1.464 billion of these sales, with the rest of the world contributing $1.228 billion. Genmab receives royalties from Janssen Biotech, Inc. on these worldwide sales under an exclusive license allowing Janssen to develop, manufacture and commercialize daratumumab.
In 2023, Darzalex reached sales of $9.744 million, marking a 22 percent increase from the previous year. This increase was attributed to gaining market share across all regions.
This upward trend is projected to continue as Darzalex remains a leading treatment for multiple myeloma, supported by its efficacy in clinical trials, broadening indications and more effective and convenient options for managing the disease.
Both Janssen and Genmab expect sustained revenue from Darzalex and Darzalex Faspro due to their established roles in multiple myeloma therapy and potential expansion in treatment protocols.
12. Eylea (Aflibercept)
Eylea 2023 Sales: $9.225 billion
Company/Developer: Regeneron
Date of First FDA approval: November 18, 2011
Indications Eylea HD Is FDA-Approved for: Neovascular (wet) age-related macular degeneration (AMD); macular edema following retinal vein occlusion (RVO); diabetic macular edema (DME) and diabetic retinopathy (DR); retinopathy of prematurity (ROP) in preterm infants
Wholesale Acquisition Cost (WAC) of Eylea HD: $2,625 per single-dose glass vial
Why It Sold So Well: For the fourth quarter of 2023, Eylea HD recorded impressive US net sales of $123 million, showcasing a strong market entry following its FDA approval in August 2023. By the end of the year, Eylea HD’s US net sales reached $166 million, underscoring a rapid adoption rate among healthcare providers and patients.
Eylea HD has likely sold well due to its extended dosing interval, which can offer significant convenience and reduced treatment burden for patients compared to the original Eylea. Peter Kaiser, MD, Chaney Family Endowed chair in Ophthalmology Research at the Cole Eye Institute and professor of Ophthalmology at Cleveland Clinic Lerner College of Medicine said in a news release on Eylea HD’s approval, “With Eylea HD, patients with wet age-related macular degeneration or diabetic retinal disease can now receive less frequent injections after their initial monthly doses and still experience the similar visual gains, anatomic improvements and safety profile of Eylea.”
Meanwhile, Eylea (the original formulation) experienced a decline in sales, with fourth-quarter US net sales amounting to $1.34 billion, a decrease from $1.50 billion in the same period the previous year. This resulted in an annual total of $5.72 billion in 2023, compared to $6.27 billion in 2022. The introduction of Eylea HD likely contributed to this shift as patients and healthcare providers opted for the newer formulation with potentially more convenient treatment schedules.
Regeneron Pharmaceuticals is currently facing legal troubles due to allegations of fraudulent drug price reporting related to its drug Eylea.
13. Trikafta (Elexacaftor/Ivacaftor/Tezacaftor)
Trikafta 2023 Sales: $8.944 billion
Company/Developer: Vertex Pharmaceuticals Inc.
Date of First FDA Approval: October 21, 2019
Indications Trikafta Is FDA-approved for: Cystic fibrosis (CF)
WAC of Trikafta: $311,741 per year
Why It Sold So Well: Trikafta is approved for the treatment of CF in patients aged two years and older who have at least one F508del mutation in the CFTR gene or a mutation in the CFTR gene that is responsive based on in vitro data.
Trikafta continued to improve product sales to $2.52 billion in the fourth quarter of 2023, up by nine percent from the fourth quarter of 2022. This is likely largely driven by the recent approval for the treatment of CF in children between the ages of two and five with certain genetic mutations, making Trikafta available to about 900 children. Along with prior approvals for CF since 2019, this expands Trikafta’s use in younger patient populations, increasing the number of eligible patients.
Clinical trials demonstrated significant improvements in lung function, reduced pulmonary exacerbations, reduction in sweat chloride and better overall health markers. Trikafta was approved in three months thanks to multiple FDA designations such as Priority Review, Fast Track, Breakthrough Therapy and Orphan Drug status, in addition to the Rare Pediatric Disease Priority Review Voucher, a new move to encourage rare pediatric disease drug development.
14. Gardasil (HPV Quadrivalent Vaccine, Recombinant; HPV 9-Valent Vaccine, Recombinant)
Gardasil 2023 Sales: $8.886 billion
Company/Developer: Merck & Co., Inc.
Date of First FDA Approval: June 8, 2006 (Gardasil); December 11, 2014 (Gardasil 9)
Indications Gardasil Is FDA-Approved for: Gardasil and Gardasil 9 vaccines are approved for the prevention of the following indications caused by human papillomavirus (HPV). Gardasil 9 is approved for use in individuals aged nine through 45 years while Gardasil is indicated for a similar range but focuses on a slightly younger demographic, up to 26 years old:
- Cancers: Prevents cervical, vulvar, vaginal, anal and certain head and neck cancers
- Genital warts: Caused by specific HPV types
- Precancerous lesions: Includes various grades of cervical, vulvar, vaginal and anal intraepithelial neoplasias
WAC of Gardasil 9: $286.78 per dose
Why They Sold So Well: Gardasil and Gardasil 9 have been highly effective in preventing infections from HPV types that cause significant health issues, including cancers and genital warts. These vaccines address the widespread prevalence of HPV and its association with various cancers and other diseases. Gardasil 9 covers the five additional HPV types beyond those initially included in Gardasil, significantly expanding its protective coverage. Since 2016, Gardasil 9 has been the only HPV vaccine approved for use in the US.
In 2023, Gardasil and Gardasil 9 sales reached almost $8.9 billion, marking a 29 percent growth from the previous year. In China, the uptake was driven by widespread vaccination campaigns and the public’s growing awareness of HPV-related cancer prevention. In the US, the growth was influenced by public-sector buying patterns and increased immunization rates. Excluding the impact of foreign exchange, Gardasil/Gardasil 9 sales grew by 33 percent.
The growth in Gardasil sales has been vital for Merck, especially as it compensates for lower sales in other segments like their antiviral drug Lagevrio (molnupiravir), which saw a decline.
Merck concluded 2023 on a high note, securing an FDA Priority Review for its investigational pneumococcal conjugate vaccine, V116, and the joint Merck-Daiichi Sankyo drug, patritumab deruxtecan. The year also saw multiple FDA approvals within its oncology portfolio and the initiation of over 20 Phase III studies, including the advancement of eight novel assets into Phase III. Additionally, Merck expanded its pipeline through acquisitions of Prometheus and Imago Biosciences, and collaborations with Daiichi Sankyo and Kelun-Biotech. Merck expects 2024 full-year overall sales to be between $63.1 billion and $64.3 billion.
Clinical trials for Gardasil 9 have shown outstanding efficacy in preventing diseases caused by the HPV types covered by the vaccine. Recently, Merck announced its plans to initiate clinical trials for a novel multi-valent HPV vaccine and to test a single-dose regimen of Gardasil 9, aiming to broaden protection against multiple HPV types and simplify vaccination schedules.
In Australia, Gardasil is funded under the National Immunization Programs for adolescents between 12 and 13 years, recognizing the importance of preventing HPV-related diseases early in life.
Under Merck’s “Merck Vaccine Patient Assistance Program,” Gardasil 9 is available for free to people in the US between the ages of 19 to 45 who lack health insurance and have a low household income.
15. Skyrizi (Risankizumab)
Skyrizi 2023 Sales: $7.763 billion
Company/Developer: AbbVie
Date of First FDA Approval: April 23, 2019
Indications Skyrizi Is FDA-Approved for: For the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy; for adults with active psoriatic arthritis; recently approved for the treatment of moderately to severely active Crohn’s disease in adults
WAC of Skyrizi: $21,017.36 per dose
Why It Sold So Well: Skyrizi, an interleukin-23 (IL-23) inhibitor, has demonstrated high clinical efficacy in the treatment of moderate-to-severe plaque psoriasis and active psoriatic arthritis. This is evident from clinical trials where 75 percent of patients achieved 90 percent skin clearance (a Psoriasis Area and Severity Index [PASI] score of 90) by 16 weeks, maintaining these results over a year. Its targeted mechanism and sustained efficacy have established Skyrizi as a preferred choice among newer biologics in the dermatology space.
In 2023, despite intense competition from biosimilars that impacted Humira’s (adalimumab) sales — especially the introduction of a higher-concentration Cyltezo (adalimumab-adbm) — Skyrizi generated $7.763 billion in global revenues. This represents a dramatic growth of over 50 percent since 2022, spearheading AbbVie’s immunology portfolio.
Further bolstering AbbVie’s position, the company’s recent acquisitions, including ImmunoGen and Cerevel Therapeutics (pending; currently under investigation), aim to strengthen its capabilities in oncology and neuroscience.
Continued investments in R&D, such as advancements with Ubrelvy (ubrogepant) — touted to raise peak sales to $3 billion with Qulipta (atogepant) in the long term — for migraine relief and lutikizumab for hidradenitis suppurativa, underscore AbbVie’s commitment to innovation. These efforts are expected to drive future growth and enhance the therapeutic value of its portfolio, aligning with the company’s vision through 2029 and beyond.
Skyrizi’s success reflects AbbVie’s ability to innovate and adapt in a changing market landscape, ensuring continued leadership in immunology and beyond.
16. Trulicity (Dulaglutide)
Trulicity 2023 Sales: $7.132 billion
Company/Developer: Eli Lilly and Company
Date of First FDA Approval: September 18, 2014
Indications Trulicity Is FDA-Approved for: As an adjunct to diet and exercise to improve glycemic control in adults and in youth and adolescents aged 10 to 17 years with type 2 diabetes inadequately controlled through diet and exercise; for reducing the risk of major cardiovascular events in adults with type 2 diabetes with known heart disease
WAC of Trulicity: $977.42 per month
Why It Sold So Well: Trulicity, a GLP-1 receptor agonist, boosts glucose-dependent insulin secretion, which is crucial for managing blood sugar levels in adults with type 2 diabetes. Type 2 diabetes affects more than 95 percent of diabetic patients. As of 2021, 537 million adults were reported to be living with diabetes, a number that is predicted to rise to 783 million by 2045.
Trulicity was approved for reducing major adverse cardiovascular events (MACE) in adults with type 2 diabetes, either with established cardiovascular disease or multiple risk factors. The approval for higher doses (3.0 mg and 4.5 mg) was followed, based on AWARD-11 trial results which demonstrated improved A1C and weight reduction. Additionally, based on the AWARD-PEDS trial, Trulicity was approved for use in youth and adolescents aged 10 to 17 with type 2 diabetes, marking a significant step for this demographic that often includes a high proportion of minority populations and presents a more aggressive disease progression.
Despite a 14 percent decrease in worldwide revenue to $1.67 billion in Lilly’s fourth-quarter (Q4) 2023 report, largely due to US market challenges like supply issues and competitive pricing pressures, Trulicity maintained strong international sales, bolstered by volume increases and favorable exchange rates. Notably, its total sales reached $7.132 billion in 2023, with projections placing Trulicity as the top diabetes drug in 2024, potentially outperforming competitors like Novo Nordisk’s Ozempic (semaglutide) and AstraZeneca’s Farxiga (dapagliflozin).
Despite facing supply constraints and competitive threats from new market entrants offering potentially superior benefits, such as Innovent Biologics’ mazdutide, Trulicity’s established efficacy, particularly in cardiovascular risk reduction, and the availability of higher dosages continue to support its widespread use.
Eli Lilly’s overall revenue surged by 28 percent to $9.35 billion in Q4 2023, driven by robust product launches and existing drug performance. With an expected revenue range of $40.4 billion to $41.6 billion for 2024, the company is strategically positioned for growth.
17. Ocrevus (Ocrelizumab)
Ocrevus 2023 Sales: $7.103 billion
Company/Developer: Genentech, a member of the Roche Group
Date of First FDA Approval: March 28, 2017
Indications Ocrevus Is FDA-Approved for: Relapsing forms of multiple sclerosis (RMS), including clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease in adults; for the treatment of adults with early primary progressive multiple sclerosis (PPMS)
WAC of Ocrevus: $78,858 per year
Why It Sold So Well: Ocrevus uniquely targets CD20-positive B cells, implicated in the myelin and nerve cell damage that characterizes multiple sclerosis (MS), reducing disease activity and progression. This was the first B-cell therapy approved for both PPMS and RMS, receiving Breakthrough Therapy, Fast Track and Priority Review designations.
In 2023, Genentech reported results from the Phase III OCARINA II study, which validated that the new 10-minute subcutaneous injection form of Ocrevus maintained efficacy comparable to the intravenous form in managing MRI lesion activity and B-cell depletion over 24 weeks. Extended data from the study indicated effective suppression of relapse activity and brain lesions through 48 weeks, supporting potential regulatory approvals expected in the US and Europe by mid-2024.
As of late 2023, Ocrevus is the most prescribed MS treatment in the US and has substantial global usage. On December 14, 2020, the FDA approved a shorter two-hour infusion time for Ocrevus, based on the ENSEMBLE PLUS study, enhancing patient convenience.
The drug’s convenience, combined with robust clinical outcomes, has broadened its appeal, leading to significant growth in both new and ongoing prescriptions.
Financially, Ocrevus was Roche’s highest-grossing pharmaceutical in 2023, with sales increasing by 13 percent over the previous year to 6.381 billion CHF (around $7.103 billion USD). This included substantial growth across all major markets: 11 percent in the US (4.684 billion CHF [$5.214 billion USD]), 12 percent in Europe (1.166 billion CHF [$1.298 billion USD], with notable upticks in Germany and Italy) and 31 percent in other international regions (531 million CHF [$591 million USD]), reflecting strong uptake in new markets.
Ocrevus’ performance significantly contributed to Roche Pharmaceuticals division’s total sales of 44.6 billion CHF ($49.6 billion USD) in 2023. Roche’s continued strategic emphasis on expanding into new therapeutic areas and enhancing neurosciences offerings is poised to solidify Ocrevus’ market position further.
18. Insulin (Various Forms)
Insulin 2023 Sales: $6.979 billion
First Company/Developer: Eli Lilly and Company
Date of First FDA Approval: Regular insulin or human insulin was approved in November 1982 (Eli Lilly and Company)
Indications Insulin Is FDA-Approved for: For daily management to maintain glycemic control in individuals with type 1 diabetes (juvenile diabetes mellitus); for use when oral medications are not sufficient to control blood sugar in type 2 diabetes; for maturity-onset diabetes of the young (MODY); for gestational diabetes as needed to control blood glucose levels during pregnancy; as a critical component in treatment protocols for diabetic ketoacidosis
WAC of Insulin: Varies significantly by type and brand of insulin — Insulin Lispro Injection 100 units/mL at $25 a vial and Lantus injection, for $92 per five pack of KwikPens
Why It Sold So Well: Insulin is essential for survival in type 1 diabetes and is crucial for many patients with type 2 diabetes, making it indispensable in diabetes management.
Insulin is normally taken using a needle and syringe, insulin pen or pump, and less commonly through inhalers (Afrezza by Mannkind) and insulin jet injectors. The FDA also approved an artificial pancreas system, called the iLet Bionic Pancreas, which uses an algorithm to regulate insulin delivery.
The first genetically modified human insulin, Humulin, was approved in 1982. Modern examples include Humalog (insulin lispro; rapid-acting insulin) and Lantus (insulin glargine; long-acting insulin), which offer better control over blood glucose levels and more convenient dosing schedules for patients.
The FDA approved the first interchangeable biosimilar insulin product, Mylan Pharmaceutical’s Semglee (insulin glargine-yfgn) in 2021. This product is clinically interchangeable with Lantus and is expected to lower the cost of insulin therapy significantly, improving accessibility and affordability for diabetes patients.
The global insulin market is estimated to grow from $19.69 billion in 2024 to $25.08 billion by 2032. This growth is driven by an increasing incidence of diabetes worldwide, increasingly sedentary lifestyles and advancements in insulin formulations.
High prices in certain markets have led to public scrutiny and legislative actions aiming to cap insulin prices. Novo Nordisk is among several insulin developers that plan to cut prices by up to 75 percent in their pre-filled pens, vials and pre-mix insulin products. In addition, emerging therapies like GLP-1 receptor agonists (like Trulicity) compete with insulin therapy.
Prominent players in the global insulin market include Eli Lilly, Novo Nordisk, Sanofi and emerging companies like Biocon and Boehringer Ingelheim. These companies’ strategic focuses include expanding into new markets, enhancing affordability and developing next-generation diabetes management solutions.
19. Imbruvica (Ibrutinib)
Imbruvica 2023 Sales: $6.860 billion
Company/Developer: LLC (an AbbVie Company) and Janssen Biotech Inc.
Date of First FDA Approval: November 13, 2013
Indications Imbruvica Is FDA-Approved for: Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL); Waldenström’s macroglobulinemia (WM); Chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy; Imbruvica was initially approved to treat mantle cell lymphoma (MCL) and marginal zone lymphoma (MZL) per Phase II studies. However, these indications were recently withdrawn after the confirmatory Phase III SHINE and SELENE trials did not meet their primary endpoints. Imbruvica continues to be approved for relapsed/refractory (R/R) MCL in over 100 countries and for R/R MZL in over 30 countries, based on positive results from earlier phase trials.
WAC of Imbruvica: Imbruvica is available in various formulations with the WAC ranging from $10,210.73 for a 70 mg/mL oral suspension to $22,690.52 for 140 mg capsules in a 120-count package.
Why It Sold So Well: Imbruvica is a kinase inhibitor that works by blocking Bruton’s tyrosine kinase (BTK), a key protein in the B-cell receptor signaling complex that plays a crucial role in the survival, proliferation and migration of malignant B cells. By inhibiting BTK, Imbruvica helps to stop the growth and spread of cancer cells.
Statista positions Imbruvica among the top ten oncology products worldwide in 2023.
Imbruvica has been a key revenue driver within AbbVie’s oncology portfolio throughout 2023 (its oncology portfolio brought in $5.915 billion in full-year net revenues for 2023), despite facing a competitive market. The drug generated global net revenues of $3.596 billion for AbbVie, a notable contribution although it represented a 21.3 percent decrease from the previous year. This decline can be attributed primarily to increased competition and market dynamics but does not detract from its strong historical performance and established efficacy. Imbruvica brought in $3.264 billion in 2023 for Janssen Biotech.
For the fourth quarter alone, Imbruvica netted $903 million for AbbVie, with a 19 percent decrease noted. The US market, contributing $683 million, continues to be the largest contributor to Imbruvica’s revenue, underscoring its importance in AbbVie’s product line despite the noted declines.
Looking ahead, AbbVie’s 2024 projections include mitigating factors from acquisitions and a strategic focus that could potentially stabilize and augment Imbruvica’s market position.
20. Xarelto (Rivaroxaban)
Xarelto 2023 Sales: $6.793 billion
Company/ Developer: Bayer AG, marketed in collaboration with Janssen Pharmaceuticals (a Johnson & Johnson company)
Date of First FDA Approval: July 1, 2011
Indications Xarelto Is FDA-Approved for: Reducing the risk of stroke and systemic embolism in individuals with atrial fibrillation; treatment and reduction in the risk of recurrence of deep vein thrombosis (DVT); treatment and reduction in the risk of recurrence of pulmonary embolism (PE); prophylaxis of DVT which may lead to PE in patients undergoing knee or hip replacement surgery; venous thromboembolism (VTE) in acutely ill medical patients at risk for thromboembolic complications who are not at high risk of bleeding; reducing the risk of major cardiovascular events such as stroke, heart attack and amputation in individuals with coronary artery disease (CAD) and peripheral artery disease (PAD)
WAC of Xarelto: $542 for a 30-day supply
Why It Sold So Well: Xarelto is an oral anticoagulant, part of the direct factor Xa inhibitors class of drugs. It has demonstrated a good balance of efficacy and safety in preventing and treating blood clot disorders. Its once-daily oral dosing simplifies treatment regimens, which is a significant advantage over traditional therapies that may require frequent dosing or monitoring, such as warfarin.
Xarelto’s continued success is driven by its extensive range of FDA-approved applications, which include the prevention of stroke in atrial fibrillation patients, treatment and prevention of DVT and PE and reduction of major cardiovascular events in patients with chronic CAD or PAD. These indications ensure its widespread clinical use across a broad patient demographic.
Xarelto continues to benefit from strong clinical backing. Recent trial data, such as from the VOYAGER PAD and PIONEER AF-PCI studies, have emphasized its efficacy and safety. These studies help reinforce the trust in Xarelto’s benefits in reducing the risk of significant adverse limb and cardiovascular events, which is crucial for healthcare providers seeking reliable anticoagulation solutions.
The drug’s performance in the first quarter of 2023 saw gains in Europe, which helped to balance some of the declines experienced in Canada, where competition with generic alternatives has intensified. In the US, where Xarelto is marketed by a subsidiary of Johnson & Johnson, there was a decrease in license revenues recognized as sales, reflecting the competitive pressures from generic products.
In 2023, Xarelto generated $4.081 billion in revenue for Bayer, making it the company’s top pharmaceutical product. Bayer’s strategic focus on growth and innovation, along with effective management of its product portfolio, ensures that Xarelto remains a key player in the market.
Despite a challenging market environment and competitive pricing pressures (especially in China and the UK), Xarelto maintained a resilient performance in Bayer’s portfolio in 2023, despite the decline since the prior-year quarter. In addition, 2024’s first quarter performance report demonstrated a 1.7 percent rise in Xarelto sales. This resilience underscores the drug’s critical role in the pharmaceutical lineup and its enduring demand.
In the past, Xarelto faced significant legal scrutiny due to allegations of severe bleeding risks. This resulted in Johnson & Johnson and Bayer agreeing to a $775 million settlement to resolve most of these lawsuits without admitting liability. The settlement, however, has not deterred the medical community from using Xarelto, given its clinical benefits and established safety profile when used as directed.
In another court ruling, the UK High Court found the dosage patent of the active ingredient rivaroxaban in Xarelto invalid due to a lack of inventive step. This decision allowed generic drug manufacturers the opportunity to enter the market, potentially reducing Bayer’s exclusive control over the drug’s sales in the region. These legal setbacks highlight the challenges Bayer faces in maintaining its market exclusivity against a backdrop of increasing generic competition.
21. Prevnar Family (Pneumococcal 7-Valent Conjugate)
Prevnar Family 2023 Sales: $6.440 billion
Company/Developer: Pfizer
Date of First FDA Approval: February 2000
Indications the Prevnar Family Is FDA-Approved for: Active immunization for the prevention of pneumonia and invasive disease caused by S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F
Cost of Prevnar 20: $261.56 per dose
Why It Sold So Well: The Prevnar Family brought in $6.440 billion for Pfizer in 2023, which accounted for 11 percent of the company’s total revenue in 2023. The FDA licensed the first pneumococcal conjugate vaccine (PCV), PCV7 or Prevnar, in 2000. That same year, the US began using PCV7 routinely in children. It provided protection against infections caused by seven types (serotypes) of pneumococcal bacteria.
In 2010, the FDA licensed PCV13 for children. This vaccine provided protection against infections caused by six more serotypes than PCV7. PCV13 caused the body’s immune system to create antibodies, which help fight pneumococcal bacteria, similar to PCV7.
In 2021, the FDA licensed PCV20 for use in adults 18 years or older, followed by approval for use in children six weeks through 17 years old in 2023. Prevnar 20 offers the broadest serotype coverage of any pediatric PCV, helping to protect against all 20 serotypes contained in the vaccine. Owing to the large impact that PCVs have had on reducing pneumococcal disease in children, the World Health Organization (WHO) has recommended the inclusion of PCVs in national immunization programs.
22. Revlimid (Lenalidomide)
Revlimid 2023 Sales: $6.179 billion
Company/Developer: BMS
Date of First FDA Approval: December 27, 2005
Indications Revlimid Is FDA-Approved for: Revlimid is a thalidomide analogue indicated for the treatment of adult patients with multiple myeloma in combination with dexamethasone; myelodysplastic syndromes; mantle cell lymphoma; follicular lymphoma; marginal zone lymphoma
Price of Revlimid: 28 oral capsules of Revlimid (2.5 mg) cost approximately $24,576
Why It Sold So Well: Revlimid is the first-line treatment for multiple myeloma and has been a star performer for BMS as it was the only treatment option available. It was also protected by patents, which gave it a great run for over a decade. Last year, the company experienced a decline in sales of Revlimid after generic versions entered the market following its patent expiration in 2022.
23. Entresto (Sacubitril/Valsartan)
Entresto 2023 Sales: $6.035 billion
Company/Developer: Novartis
Date of First FDA Approval: July 7, 2015
Indications Entresto Is FDA-Approved for: Entresto is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. It is also indicated for the treatment of symptomatic heart failure with systemic left ventricular systolic dysfunction in pediatric patients aged one year and older.
List price of Entresto: $688.01 a month
Why It Sold So Well: Entresto was the first drug approved in its class to treat patients with chronic heart failure with reduced ejection fraction. It became a cornerstone of treatment for heart failure patients after the American College of Cardiology (ACC)/American Heart Association (AHA) gave Entresto Class 1 recommendation in 2016.
Given its success rate and the nonavailability of generics, Entresto has generated great revenue for Novartis year over year. It is patent-protected until 2025. However, the FDA has recently approved generics that will be available once the patent expires, which will affect its future revenues.
24. Xtandi (Enzalutamide)
Xtandi 2023 Sales: $5.971 billion
Company/Developer: Pfizer/Astellas Pharma
Date of First FDA Approval: August 31, 2012
Indications Xtandi Is FDA-Approved for: Prostate cancer that hasn’t spread, or metastasized, to other parts of the body. Xtandi is also used for prostate cancer that has spread to other parts of the body and is responding to hormone therapy.
List price of Xtandi: $14,322.47 per month
Why It Sold So Well: First approved by the FDA in 2012 for metastatic castration-resistant prostate cancer (mCRPC), Xtandi has become a standard treatment across various stages of this cancer type.
In 2018, Xtandi received approval for non-metastatic castration-resistant prostate cancer, and in 2019, for metastatic castration-sensitive prostate cancer.
It gained another approval in November 2023 for non-metastatic castration-sensitive prostate cancer with biochemical recurrence at high risk for metastasis. The new approval has made Xtandi the first and only androgen receptor signaling inhibitor approved for use without a gonadotropin-releasing hormone therapy in nonmetastatic castration-sensitive prostate cancer.
Xtandi is still creating significant revenue and is patent-protected until August 2027 in the US, which shields it from generic drugs.
25. Farxiga (Dapagliflozin)
Farxiga 2023 Sales: $5.963 billion
Company/Developer: AstraZeneca
Date of First FDA Approval: January 13, 2014
Indications Farxiga Is FDA-Approved for: For adults with chronic kidney disease (CKD) to lower the risk of persistent decline in estimated glomerular filtration rate (eGFR), end-stage kidney disease and both cardiovascular and renal death; to reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure with reduced ejection fraction; to reduce the risk of hospitalization for heart failure in adults with type 2 diabetes and established cardiovascular disease or multiple cardiovascular risk factors; to improve blood sugar control along with diet and exercise in adults with type 2 diabetes as part of combination therapy
List Price of Farxiga: $582.25 for a 30-day supply
Why It Sold So Well: Farxiga is a pioneering oral, once-daily sodium-glucose cotransporter-2 (SGLT2) inhibitor. Research on Farxiga is progressing beyond its cardiorenal effects to explore its potential in prevention and organ protection, as ongoing studies reveal the interconnected roles of the heart, kidneys and pancreas.
Farxiga sales soared after its approval for use in patients with heart failure and kidney disease. For nearly a decade, Farxiga has proven effective as both a monotherapy and as part of combination therapy, complementing diet and exercise to enhance glycemic control in adults with type 2 diabetes.
26. Tagrisso (Osimertinib)
Tagrisso 2023 Sales: $5.799 billion
Company/Developer: AstraZeneca
Date of First FDA Approval: November 13, 2015
Indications Tagrisso Is FDA-Approved for: Tagrisso is a prescription medicine used to treat adults with NSCLC that has certain abnormal epidermal growth factor receptor (EGFR) gene(s).
Price of Tagrisso: $18,034 for a supply of 30 tablets
Why It Sold So Well: Tagrisso is a third-generation, irreversible EGFR-tyrosine kinase inhibitor (TKI) known for its clinical efficacy in treating NSCLC, including cases with central nervous system metastases. Available in 40 mg and 80 mg once-daily oral tablets, Tagrisso has been administered to over 800,000 patients globally across its approved indications.
AstraZeneca continues to investigate Tagrisso’s potential as a treatment for patients at various stages of metastatic EGFR-mutated NSCLC. Tagrisso is approved as a monotherapy in over 100 countries, including the US, European Union (EU), China and Japan.
27. Botox (OnabotulinumtoxinA)
Botox 2023 Sales: $5.673 billion
Company/Developer: AbbVie
Date of First FDA Approval: December 29, 1989
Indications Botox Is FDA-Approved for: Botox is an acetylcholine release inhibitor and neuromuscular blocking agent indicated for chronic migraine, urinary incontinence and overactive bladder, cervical dystonia, neurogenic detrusor overactivity, spasticity, severe axillary hyperhidrosis, and for cosmetic purposes (Botox cosmetic)
WAC of Botox: Cost depends heavily on the condition and dose required, but the list price is $1,244 for a 200-unit vial.
Why It Sold So Well: Botox is FDA-approved for the treatment of migraines, the only chronic condition for which it has received approval. Botox is a biologic drug available exclusively as a brand-name medication; it is not currently available in generic or biosimilar forms.
Moreover, Botox is one of the most prescribed branded treatments for chronic migraine prevention, offering quick relief with just four treatments a year.
28. Entyvio (Vedolizumab)
Entyvio 2023 Sales: $5.280 billion
Company/Developer: Takeda
Date of First FDA Approval: May 20, 2014
Indications Entyvio Is FDA-Approved for: Ulcerative colitis in adults and Crohn’s disease in adults
WAC of Entyvio: One vial of an intravenous solution reconstituted (300 mg) is $8,666.58 and one pen of a subcutaneous solution pen-injector (108 mg/0.68mL) is $3,119.97.
Why It Sold So Well: Entyvio was approved by the FDA for the treatment and maintenance of patients with ulcerative colitis, but it has garnered additional approvals for subcutaneous use.
The Entyvio pen is also available, which offers flexible maintenance and ease of at-home use.
Globally, Entyvio has more than one million patient years of exposure to date.
29. Mounjaro (Tirzepatide)
Mounjaro 2023 Sales: $5.163 billion
Company/Developer: Eli Lilly and Company
Date of First FDA Approval: May 13, 2022
Indications Mounjaro Is FDA-Approved for: Mounjaro is used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
List Price of Mounjaro: $1,069.08 per fill
Why It Sold So Well: Tirzepatide, the first and only dual-acting glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist, is approved for type 2 diabetes. It uniquely targets the GIP receptor, enhancing insulin regulation and blood sugar control similar to GLP-1. In November 2023, tirzepatide, marketed as Zepbound, also received FDA approval for chronic weight management in adults with obesity.
30. Cosentyx (Secukinumab)
Cosentyx 2023 Sales: $4.980 billion
Company/Developer: Novartis
Date of First FDA Approval: January 21, 2015
Indications Cosentyx Is FDA-Approved for: Plaque psoriasis; psoriatic arthritis; active ankylosing spondylitis; enthesitis-related arthritis; non-radiographic axial spondyloarthritis; hidradenitis suppurativa
List Price of Cosentyx: $7,408.96 a month for either a 150-mg or a 300-mg dose strength self-injection package and $4,230 per intravenous infusion
Why It Sold So Well: Since its initial approval in 2015, Cosentyx has demonstrated sustained efficacy and a consistent safety profile across five systemic inflammatory conditions, treating over one million patients worldwide. Its diverse range of therapeutic indications, namely expansion into inflammatory conditions beyond psoriasis, has aided in higher sales.









Join or login to leave a comment
JOIN LOGIN