A vaccine developed by Swedish company Diamyd Medical has demonstrated significant treatment efficacy in a predefined genetic subgroup of individuals with type 1 diabetes in a Phase IIb clinical trial.
The predefined genetic subgroup consists of patients positive for the human leukocyte antigen (HLA) DR3-DQ2 haplotype, which is found in approximately 50 percent of all type 1 diabetics worldwide. Targeting such a major proportion of disease sufferers is significant, positioning the vaccine to be a potential game changer for this population.
Diamyd Medical is a clinical-stage biopharmaceutical company that develops therapies for type 1 diabetes. In addition to the vaccine, the company is also developing an investigational drug called Remygen for the regeneration of endogenous insulin production and to improve hormonal response to hypoglycemia; trials to test the drug are currently ongoing in patients that have been living with type 1 diabetes for more than five years.
Based on the promise of the topline Phase II results for its vaccine, Diamyd Medical says it will pursue the HLA restricted responder subgroup in an upcoming pivotal Phase III program.
Related: Medtronic Introduces New Minimed 770G For Type 1 Diabetes
In the trial, the vaccine was injected directly into the lymph nodes of type 1 diabetic individuals. The trial showed a preservation of function of insulin-producing β-cells at 15 months post-diagnosis. Encouragingly, no serious adverse events associated with the vaccine were observed.
The results served to validate a large-scale retrospective meta-analysis that Diamyd Medical had conducted previously, with findings from it published recently in the journal Diabetologia. The results of the analysis showed that individuals positive for the HLA DR3-DQ2 haplotype demonstrated favorable responses to the vaccine, identifying a genetically-predefined subset of type 1 diabetics that can be treated with the antigen-targeted vaccine.
How the Type 1 Diabetes Vaccine Works
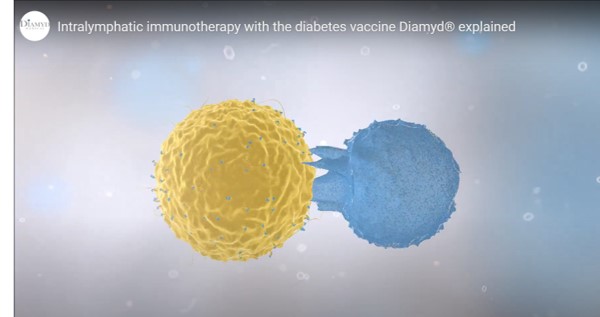
The vaccine – trademarked as Diamyd – is an antigen-specific immunotherapy that helps to preserve endogenous insulin production. The vaccine triggers an immune response against pro-inflammatory processes resulting from autoimmune attack of the pancreas – the inflammation helps to destroy the insulin-producing β-islet cells of the organ, giving rise to the disease.
Specifically, the vaccine contains human recombinant glutamate decarboxylase 65 (GAD65) protein conjugated to aluminum hydroxide (GAD-alum). GAD65 is an enzyme that catalyzes the decarboxylation of glutamate to gamma-aminobutyric acid (GABA) and CO2. GAD exists in two isoforms in mammals, distinguished by a difference in their molecular weights of 67 and 65 kDa (GAD67 and GAD65, respectively).
In addition to being expressed in the brain where GABA is used as a neurotransmitter, GAD67 and GAD65 are also expressed in varying ratios in the β-cells of the pancreas. Immune cells express GABA receptors, which when activated by the binding of GABA, can suppress inflammatory responses and promote “regulatory” immune responses. In fact, GABA administration has been shown to inhibit autoimmune diseases in several animal models.
Therefore, the rationale for targeting GAD65 in the pancreas is to program immune cells into therapeutically active anti-inflammatory cells to prevent destruction of pancreatic insulin-producing β-cells (see video above).
The DIAGNODE-2 Trial
The Diamyd GAD vaccine was evaluated in a double-blind, placebo-controlled, European phase IIb trial called DIAGNODE-2. The trial included 109 patients aged 12 to 24 years from 18 clinics in Spain, the Czech Republic, Sweden and the Netherlands. The patients in the trial had new-onset type 1 diabetes, having been diagnosed within six months of therapeutic intervention, along with evaluation of fasting C-peptide; C-peptide is a byproduct of insulin production and serves as a surrogate for evaluating insulin production status in the body.
The patients were given three doses of the diabetes vaccine, or placebo, at one-month intervals, administered directly into a lymph node through ultrasound guidance. The trial participants were also given oral vitamin D (or placebo) supplements for four months, with vitamin D supplementation starting 30 days before the first injection of Diamyd.
The patients were followed for 15 months in order to evaluate endogenous insulin production, as measured by C-peptide. Of the initial 109 patients who entered the trial, 107 patients completed all visits up to 15 months; only two patients chose to terminate the trial prematurely.
The primary endpoint of the trial was preservation of β cell function at 15 months, as measured by meal stimulated C-peptide. Of the 103 patients evaluated, 55 participants received the vaccine while 48 received placebo. Of the 103 patients, 46 were positive for the HLA DR3-DQ2 haplotype and 29 of them received active treatment while 17 received placebo.
According to a press release from Diamyd yesterday, in which they outlined results from the trial, a statistically significant (p < 0.01) treatment effect was observed in the predefined subgroup of patients positive for HLA DR3-DQ2. However, overall, a limited positive, but non-significant treatment effect was observed across the total cohort of 103 patients that were evaluated.
In an online press conference held yesterday, Dr. Ulf Hannelius, CEO of Diamyd, outlined the three main findings from the trial, which were:
- A treatment effect of more than 50 percent greater preservation of endogenous insulin production compared to placebo in HLA DR3-DQ2 positive individuals
- Positive trends in important secondary endpoints (change in HbA1c – indicator of blood glucose control in patients – as well as insulin dose and insulin-adjusted HbA1C)
- No safety concerns
Hannelius said, “The results validate our previous large-scale retrospective meta-analysis that we recently published, [which] showed that when you treat a type 1 patient with the GAD therapeutic, and when you take into consideration the HLA genotype, you see that you get a very good response in individuals who are positive for the HLA DR3-DQ2 haplotype. So, this really validates what we already knew and now we have prospective results supporting that [from this trial].”
Hannelius said this highlights the importance of genetics when developing therapies for type 1 diabetes. “It shows the importance of taking into consideration the heterogeneity of the disease and explains why so many trials in the field have shown conflicting results before.”
Moreover, in terms of its safety profile, Hannelius explained that the therapeutic is not immunosuppressive, so there is not an increased infection risk for patients when they are treated with the vaccine.
Dr. Johnny Ludvigsson, Professor at Linköping University and Coordinating Investigator of the trial said he is “overjoyed by the positive results.” He explained, “What we see now is that the GAD treatment works for nearly half of the patients with type 1 diabetes. It is important that this patient group may soon benefit from an effective, safe and convenient treatment that does not suppress the immune system, especially now in the era of COVID-19.”
Hannelius said as a company, the results provide a clear path forward in developing a therapy that addresses a very large portion of individuals with type 1 diabetes.
“Supported by these positive results and the superior safety profile, we will now move forward with a Phase III program in this patient population, representing close to one-half of all individuals with type 1 diabetes.”








Join or login to leave a comment
JOIN LOGIN