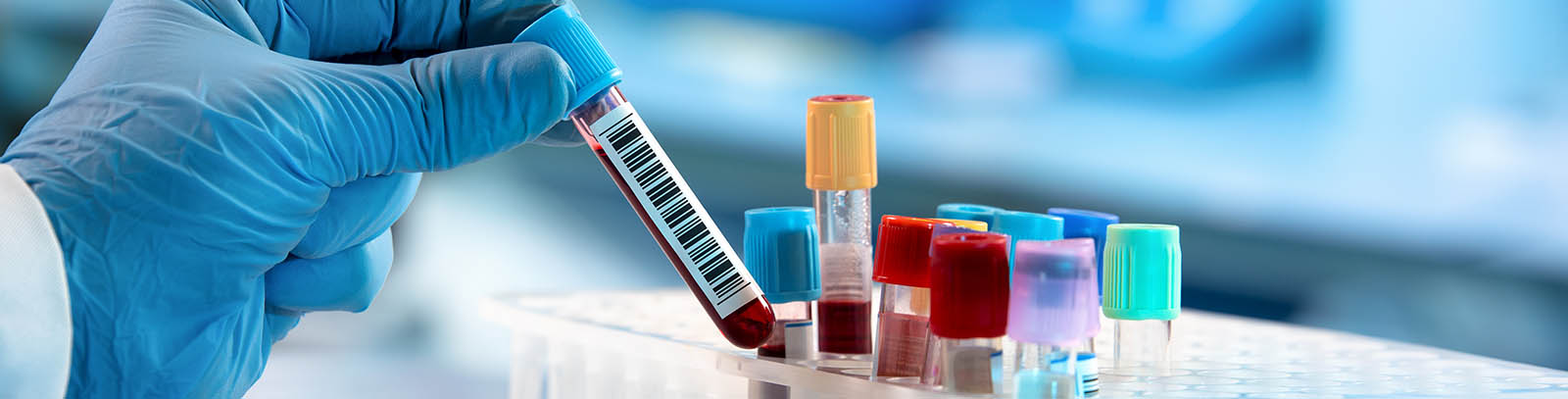
Patient samples are collected, and now it’s essential to make sure they get where they’re supposed to go. A lab may have routine studies or a fair share of complex trials, regardless, these studies are always going to come with strict timelines and clinical sample management requirements. Additionally, when global logistical needs are added, there’s very little room for error.
Often, the unexpected does happen that may cause unforeseen logistical challenges. Expecting the unexpected and planning appropriately ensures reliable and timely delivery of clinical samples.
Read more...
Most Phase II and Phase III studies have multiple endpoints and procedures per patient generating millions of data points from a single study. And with the exponential rise in technology and capability, these numbers are only going to go up. Not only is this more burdensome for patients, and sites to recruit patients, but it increases the complexity of the logistics and sample handling for trials since samples comprise most data for any submission.
Therefore, the question raised is: How to ensure the labs working on these increasingly complex studies aren’t getting bogged down, and are they moving fast enough for the lives that depend on them?
Read Less...
This webinar is part of a series addressing key challenges facing sponsors of clinical trials.
Join this webinar to gain valuable insights into effective clinical sample management techniques and essential precautions to prevent sample loss. Attendees will explore the realm of sample handling, agile shipping strategies, digital technology for ensuring a robust chain of custody and the optimal solutions for maintaining the clinical sample integrity during storage.
Speakers

Stephanie Weber, Vice President, Sample Tracking, SampleGISTICS, LabConnect
Stephanie Weber joined LabConnect in 2009 after more than 10 years of combined central and specialty laboratory project management experience across domestic and global clinical trials. She is responsible for the management and oversight of LabConnect’s sample management and tracking services on a worldwide basis. Stephanie has spent the last decade working with top pharma clients as well as other industry experts to help develop LabConnect’s comprehensive sample tracking platform, SampleGISTICS™. She has a degree in chemistry from the College of Staten Island as well as a degree in law from John Jay College of Criminal Justice. Stephanie currently resides in Washington State.

Joe Marino, Senior Director, FSP Solutions, LabConnect
Joe Marino is the Senior Director of FSP Solutions at LabConnect. Joe brings over 18 years of experience in managing complex analytical testing profiles and research vendors for the development of novel therapeutics. He is responsible for leading a team of scientists, sample management professionals and contract managers strategically supporting clients within the pharmaceutical industry. He also has extensive experience in leading activities in method development and validation, process development, quality control and analytical lifecycle management. During his time at LabConnect, Joe has been responsible for the scientific oversight and clinical phase project management of numerous pharmaceutical products. In addition to his analytical expertise, Joe has specialized in the development of processes to standardize collection and isolation procedures for blood derived components. Joe holds a BS in microbiology from Washington State University and a MS in microbiology from the University of Hawaii.

Julia Tarasenko, Senior Vice President, Global Commercial Operations, Sales and Pricing, Marken
Julia Tarasenko is SVP, Global Commercial Operations, Sales and Pricing at Marken and has been a valuable member of Marken since 2008, holding diverse leadership positions within the company. Her roles have encompassed managing European client relationships, overseeing operations in Europe and the APAC region and serving as a regional director in North America. Julia’s contributions have been instrumental in expanding the APAC network, strengthening key client relationships and driving strategic global partnerships.
Presently, she leads Marken’s global sales and commercial operations, where she oversees pricing teams worldwide and the team of global alliance directors. As a member of the Marken Executive Committee, Julia actively supports the organization’s operations, global expansion and innovative direction to ensure Marken continues to provide impactful solutions worldwide.
Who Should Attend?
- Senior professionals working in translational medicine
- Clinical Operations Team Managers and Executives
- C Level Managers
What You Will Learn
Attendees will gain insights into:
- Agile approaches to clinical sample shipping strategy
- Digital technology options for ensuring a solid chain of custody
- Best solutions for maintaining the integrity of clinical samples while they are in storage
Xtalks Partner
LabConnect
LabConnect improves lives by partnering with pharma/biotech companies and clinical research organizations (CROs) to accelerate the development of medicines around the world. An independent, global, one-stop-shop focused on delivering central laboratory services that are tailor-made, timely and flexible to meet the evolving study demands of traditional and increasingly complex trials. With deep and unmatched experience, LabConnect’s team of scientific experts serves as an extension of our clients’ clinical teams, quickly coordinating all laboratory related needs and providing end-to-end analytical and logistical solutions.
Leading the evolution in central laboratory services since 2002, our services are tailored to fit the unique needs of your trial. Connect with us at www.labconnect.com or via email at [email protected].
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account