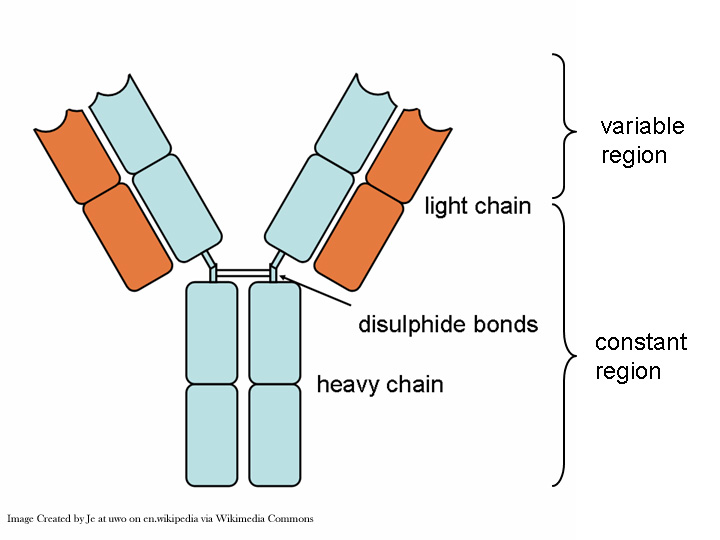
Precision medicine promises tailored treatment for patients and significantly reduced drug failure rates for biopharmaceutical companies. In order to deliver targeted therapeutics, drug developers must design clinical trials that utilize a validated companion diagnostic assay as part of the enrollment criteria, an increasingly difficult prospect as the field moves beyond assays that simply determine the presence of the therapeutic target molecule.
Myriad Genetics is uniquely positioned to provide support to pharmaceutical and biotechnology companies via high-complexity diagnostic platforms for DNA, RNA, and protein measurements, vertically-integrated biomarker discovery, clinical validation, and diagnostic commercialization capabilities, all of which are backed by a regulatory infrastructure that achieved the industry’s first FDA approval for a complex companion diagnostic.
Attend this webinar to learn more about how the largest molecular diagnostic clinical laboratory in the world can help identify molecular characteristics linked to complex disease states and drug response phenotypes to improve drug development and help deliver the promise of precision medicine.
Speaker

Patrick M. Burke, Ph.D. ,Executive Vice President, Emerging Products, Myriad Genetics
Patrick Burke, executive vice president of emerging products, joined Myriad Genetics, Inc. in 2001. In his current role, he oversees New Product Planning, Business Development and Project Management. Previously, Dr. Burke served as Vice President of Strategic Collaborations and held positions of increasing responsibility within the Business and Corporate Development group at Myriad. Between 2009 and 2011, he served as Vice President of Corporate and Business Development at Myrexis, Inc., a subsidiary of the company. Dr. Burke is an active member of Licensing Executive Society and the Association of University Technology Managers. He earned his Ph.D. in Cell Biology from the University of Utah School of Medicine and his B.A. in Molecular Biology from the University of California, San Diego.
Who Should Attend?
Clinical and scientific leaders involved in personalized medicine strategies and implementation for drug development programs.
Individuals and teams tasked with incorporating hereditary and somatic gene mutations, gene expression, and blood-based protein biomarkers in CDx programs, especially in oncology and inflammatory and autoimmune disorders.
Xtalks Partner
Myriad
Myriad RBM, Inc. is the world’s leading multiplexed immunoassay testing laboratory, providing comprehensive protein biomarker services based on its Multi-Analyte Profiling (MAP) technology platform. This platform provides pre-clinical and clinical researchers with reproducible and quantitative data for a few or hundreds of proteins in a cost-effective manner. All services are performed in our CLIA certified laboratory. As a guide to drug development researchers, Myriad RBM also offers Strategic Biomarker Services that include companion diagnostics, custom assay development, co-sponsored research programs, and innovative cell culture products. Myriad RBM’s biomarker testing laboratory is located in Austin, TX. Myriad RBM is a wholly owned subsidiary of Myriad Genetics, Inc. (MYGN), a leading molecular diagnostic company based in Salt Lake City, Utah which develops and markets novel predictive medicine, personalized medicine and prognostic medicine tests.
Media Partner
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account