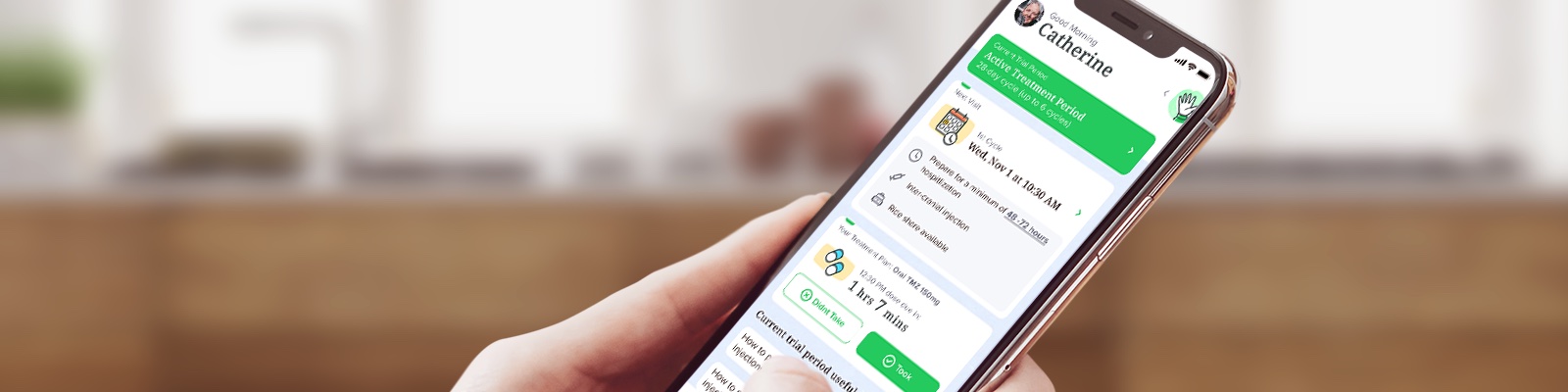
Patient satisfaction and engagement are key to achieving retention and compliance in cell and gene therapy trials, where commitment is crucial, from cell harvesting to ensuring patient involvement in lymphodepletion and study-specific procedures.
Join the featured speakers for an insightful panel discussion on empathetic engagement: a study companion app that transforms the patient experience of these complex cell and gene therapy trials. A concept that combines in-depth therapeutic and operational experience, with engaging, cutting-edge technologies for patients across therapeutic areas. With personalized features, such as direct messaging with study sites, medication and appointment reminders, engaging push notifications and connecting a patient’s support network, study companion apps are creating a seamless and connected journey for patients, from the moment their trial begins.
In collaboration with GRYT Health, a patient panel was conducted, gathering feedback from experienced clinical trial patients who assessed StudyKIK’s oncology app. Discover the remarkable insights gained from participants aged 35 to 76, selected for their demographics and familiarity with adoptive cellular therapies.
Join Greg Christie, Jane Bentley and a member of this patient panel, as they delve into the insights on how use of a study companion app can impact the clinical trial experience for patients who participate in complex oncology trials. This discussion will explore participants’ comfort levels with app usage, their desires, hesitations and invaluable feedback on the user experience.
Don’t miss this exclusive opportunity to register and unlock the true potential of study companion apps, redefining the intricate realm of cell and gene therapy trials and fostering meaningful patient experiences in the world of tech-driven research.
Speakers

Greg Christie, Chief Product Officer, StudyKIK
Greg Christie is a renowned thought leader in human-centered product development and design. As the Chief Product Officer at StudyKIK, he has gained recognition for his contributions to clinical research technology. Greg’s work on the oncology app concept earned StudyKIK the SCOPE Participant Engagement Award, emphasizing his commitment to a collaborative and user-focused approach that prioritizes participant experience in cell and gene therapy trials.
Under Greg’s leadership, StudyKIK was selected as a finalist for the esteemed Prix Galien UK 2023 Award in the ‘Best Digital Health Solution’ category. This distinction celebrates the patient-centric design and transformative impact of StudyKIK’s study companion applications across various therapeutic areas, including immunology and women’s health.
Greg recently received the prestigious 2023 PM360 ELITE 100 Award in the Tech-Know Geek category for his contributions in reshaping the medtech landscape through innovative and patient-centric products.
Guiding StudyKIK’s Product and Design teams, Greg brings a wealth of experience from his previous roles at technology organizations such as Goldstar Events, Evite and prominent publications including Elle, Woman’s Day, Car and Driver, Road & Track and Popular Photography. His expertise spans product development, design, branding and marketing, positioning him as a versatile and influential leader in the field.

Jane Bentley, Vice President, Oncology Therapeutic Strategy and Innovation, Syneos Health
In her role as an Innovation and Strategy lead for Oncology at Syneos Health, Jane Bentley leads the Syneos Health Oncology initiatives in advanced cell therapy, including CAR T therapies in collaboration with GRYT Health and the development of a new patient ad board solution (CRAFTT Express) for Oncology Biotech customers. Jane is an active participant in Syneos Health’s cross functional consortia for patient voice, cell and gene therapy and rare disease. She has over 30 years of global oncology clinical research expertise in pharma/CRO, including 20 years leadership experience in oncology global trial management across Phases I-IV. Before joining Syneos Health Jane held oncology leadership roles in several global CROs and consultancy organizations. Her experience in global trial management includes key oncology/hemato-oncology indications (breast cancer melanoma, glioblastoma, prostate cancer, multiple myeloma, AML, supportive care neutropenia/anemia) including immunotherapies and biologicals and across Phases I-IV. Her experience also includes global leadership of registration programs and support of clients in the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) inspection readiness.
She is currently a member of the board at the Institute of Clinical Research and previously held the post of Hon. Lecturer in Clinical Research at Liverpool John Moores University. Jane holds a BSc in pharmacology and a PhD in toxicology from the University of London. She is currently close to completing her study towards an MBA in International Health Medicine.

Joel Santos Gonzalez, Intensive Reading Teacher, Wendell Krinn Technical High School
At 34 years old, Joel Santos Gonzalez is a two-time cancer survivor. He had Hodgkin Lymphoma from 2017-2019 and again from 2020-2021. Joel is presently in remission. As a cancer survivor, he cares a lot about helping others deal with cancer and doing what he can to give back. He is an Intensive Reading Teacher at Wendell Krinn Technical High School.
Who Should Attend?
Pharmaceutical and Biotech personnel with the following departments and/or job titles:
- C-Suite
- VP Level
- Clinical Development Director
- Director, Clinical Research
- Director, Procurement
- Director, Outsourcing
- Digital Innovation
- Decentralized Trials
- Patient Solutions
- Patient Engagement
What You Will Learn
Attendees will:
- Understand patient comfort levels and prior experience with apps, both in general and in the context of healthcare and clinical trials
- Identify patient preferences and desires regarding the features and functionalities they would like to see in a clinical trial app, while also recognizing their hesitations or concerns
- Gather valuable feedback from patients who have participated in a prototype clinical trial app, providing insights into its effectiveness and usability
Xtalks Partner
StudyKIK
StudyKIK, a Syneos Health Company, is at the forefront of clinical trial management technologies, specializing in innovative solutions for patient recruitment and retention. Since its establishment in 2014, StudyKIK has been dedicated to enhancing and optimizing the clinical trial lifecycle, redefining the experience for patients, sites, and sponsors alike.
You Must Login To Register for this Free Webinar
Already have an account? LOGIN HERE. If you don’t have an account you need to create a free account.
Create Account