Women encounter specific health issues that require specialized research and healthcare solutions, ranging from breast cancer to endometriosis. Despite advancements in medical research, women’s health has historically been underrepresented and underfunded, leading to disparities in treatment efficacy and safety for women.
“Failure to study medications and other interventions in a broad sampling has led to women experiencing adverse effects from medications at nearly twice the rate of men,” says Dr. Jaclyn Jansen, MD, MS, Medical Director of the Medical Department, General Medicine, Medpace. This is partly due to dosages and treatment regimens traditionally being based on data from male-dominated studies.
In recent years, the landscape of women’s health clinical trials has begun to shift. There is a growing recognition of the need for inclusive research that accurately reflects the diverse experiences of women.
“Biologic sex can play a role in physiologic, metabolic, hormonal and even cellular differences when it comes to the therapeutic response between women and men.”
— Jaclyn Jansen
Dr. Jansen recently moderated a webinar in which her colleagues at Medpace discussed the evolving women’s health sector, including the historical challenges and emerging trends in clinical development. In the webinar, Dr. Aparna Shah, MD, FACOG, Medical Director of the Medical Department, Women’s Health at Medpace, shared insights about where the focus on women’s health has traditionally been and why this may be evolving.
Dr. Shah was joined by her colleague Dr. Rujin Ju, MD, MSCR, FACOG, Medical Director of the Medical Department, Women’s Health at Medpace, who discussed innovation in women’s health clinical research and FemTech — innovative technology-based products and services designed to address women’s health issues.
Beth Tulip, Senior Executive Director of Clinical Trial Management at Medpace, also participated and shared key operational strategies for women’s health clinical trials.
Read on to learn more about the historical challenges, recent advancements, clinical considerations, and the future of women’s health clinical trials, as detailed by Medpace’s women’s health experts.
Unique Challenges in Women’s Health Clinical Development
Understudied, Underfunded, Underrepresented
Recent research and historical data increasingly highlight the unique health challenges faced by women, revealing significant gender disparities in medical outcomes and treatment effectiveness. For example, a 2013 study found that women were 29 percent more likely to experience implant failure of metal hip replacements compared to men.
Additionally, the presentation of diseases can vary greatly between genders. For instance, sexually transmitted infections (STIs) can have different long-term repercussions for women as compared to men. Another example is heart disease, the leading cause of death for both men and women in the US. It wasn’t until 1999, when the American Heart Association released its Guide to Preventive Cardiology for Women, that the medical field acknowledged that women show different symptoms of the disease than men.
Health disparities add to these challenges. Cultural, social and economic factors contribute to gender-based gaps in healthcare. Women, particularly those from marginalized communities, often face barriers to accessing quality healthcare, leading to disparities in diagnosis, treatment and outcomes. For instance, women of color are at a higher risk of maternal mortality and morbidity due to a combination of systemic biases and socioeconomic factors.
The Under-Representation of Women in Clinical Trials
Although women make up about half of the global population, clinical research has historically focused primarily on men. This disparity is even more pronounced among pregnant women, who are notably underrepresented in research. Several factors contribute to this under-representation:
- Historical Exclusion: The thalidomide tragedy of the 1950s and 60s, where a drug used to treat nausea in pregnancy caused severe birth defects, prompted the US Food and Drug Administration (FDA) to implement strict policies. In 1977, the FDA excluded women of childbearing potential from early-phase clinical trials to protect pregnant women and their unborn babies. This policy led to a significant data gap in women’s health clinical research.
- Misconceptions and Biases: Despite the known physiologic, metabolic and hormonal differences between men and women, there remains a long-standing misconception that drugs exert the same effects in both men and women. For instance, the same drug may be less effective or cause more side effects in women than in men. The concern that study outcomes may be confounded by fluctuating hormone levels in women have also contributed to the under-representation of women in studies.
- Ethical and Practical Concerns: The unique ethical considerations that accompany pregnant women in trials have also limited their participation. Additionally, due to the greater share of household and childcare responsibilities shouldered by women compared to men, women encounter more practical barriers in clinical trials. These barriers include the need for flexible trial hours, childcare, and travel provisions, which are frequently not adequately addressed in study designs.
- Regulatory and funding gaps: The historical lack of funding and regulatory support for women’s health research has undoubtably contributed to the paucity of studies that address female-specific conditions or gender differences in treatment responses.
Recognizing these issues is the first step towards change.
Policy and Funding Shifts that have Supported Women’s Health Clinical Trials
Women’s Health – On the Upswing in 2024
Key policy changes have supported women’s health clinical research over the past few decades. A major turning point was The NIH Revitalization Act of 1993 which established guidelines for the inclusion of women and underrepresented racial and ethnic minority populations in clinical research. This policy shift was supported by the creation of the Office of Research on Women’s Health (ORWH) at the NIH and the FDA’s Office of Women’s Health, both aimed at advancing gender equity in clinical studies.
In 2016, the FDA’s Center for Devices and Radiological Health (CDRH) established the Health of Women Program to study issues related to the performance of medical devices in women, highlighting the necessity of gender-specific data.
Historically underfunded, women’s health clinical research received a major boost in February 2024 with a $100 million federal commitment towards transformative R&D. This significant investment aims to drive innovation and address long-standing gaps in women’s healthcare.
The private sector has also shown increasing interest in women’s health. Since 2020, private equity investment in women’s health has surged. Despite a challenging fundraising environment in 2023, investment in women’s health saw a remarkable 300 percent increase compared to 2020. This surge reflects a growing recognition of the market potential in addressing women’s health.
Several factors have contributed to this increased interest and resultant capital influx. One major driver is that employed women spend approximately $15 billion more in annual out-of-pocket healthcare costs compared to employed men. Additionally, cultural shifts have led to more open discussions about previously taboo subjects like menopause and sexual health, creating new opportunities for investment and innovation.
Expanding Focus Beyond Mortality
Defining Women’s Health
Dr. Shah emphasized that traditional women’s health research has primarily targeted conditions with high mortality rates, such as ovarian, cervical and uterine cancers. Conditions like endometriosis, fibroids and menopause, which cause chronic pain and other severe symptoms that significantly impact quality of life, have historically received less attention and funding.
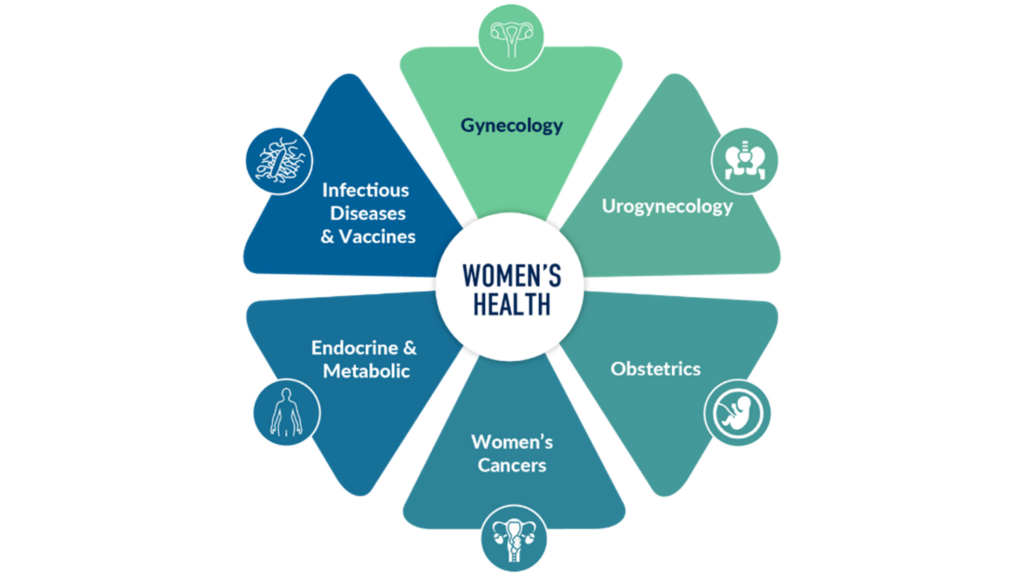
Figure 1. Defining women’s health — Medpace’s diverse therapeutic expertise.
Menopause can severely impact well-being and productivity, with menopausal symptoms impacting a majority of women at some point during the menopausal transition and beyond. Symptoms such as hot flashes and mood changes contribute to nearly $2 billion in annual lost work productivity. Innovative solutions to manage these symptoms could greatly improve the quality of life for women.
“I think we are starting to recognize that conditions like endometriosis, female sexual dysfunction, vasomotor symptoms and genitourinary syndrome of menopause, which can have a hugely negative impact on quality of life and currently have inadequate treatment options, really deserve more attention.”
— Aparna Shah
Endometriosis, affecting about 10 percent of women of reproductive age, causes severe pain and infertility. Current treatments are often inadequate, highlighting the need for new therapies that can better manage pain and improve fertility outcomes.
Fibroids, non-cancerous growths in the uterus, can cause heavy menstrual bleeding, pain and reproductive issues. Despite being very common, affecting up to 80 percent of black women and 70 percent of white women by age 50, fibroids have not received the research attention they deserve.
The evolving focus in women’s health aims to address these gaps by also prioritizing conditions that primarily affect quality of life. This includes developing new therapies and improving existing ones for chronic conditions long neglected. Rising investment in R&D for conditions like endometriosis and menopause reflects this shift.
Opportunities for Innovation in Women’s Health
Dr. Ju emphasized the potential for innovation in women’s health beyond the traditional focus on fertility, pregnancy and oncology. Conditions like menopause and endometriosis present opportunities due to their high prevalence and the current lack of effective treatments.
“Investors are recognizing the untapped potential and significant opportunities to fill healthcare gaps for women… across their entire life journey.”
— Rujin Ju
There is also growing interest in sexual health, pelvic health and contraception. Innovations in these areas can provide women with more effective and personalized health options. For example, advancements in non-hormonal contraceptive methods can provide women with more options that align with their individual health needs and preferences.
Technological advancements are driving innovation in women’s health through FemTech, including menstrual tracking apps, ovulation monitors and wearable devices. These technologies offer valuable health insights and generate data for further R&D.
Collaborations between tech companies and healthcare providers are leading to new, integrated solutions that combine digital health tools with medical treatments. This approach can enhance treatment effectiveness, improve patient adherence and provide real-time health monitoring, leading to better health outcomes.
The Rise of FemTech

Technological advancements are driving innovation in women’s health through the rise of FemTech. This sector, encompassing menstrual tracking apps, ovulation monitors and wearable devices, is transforming how women manage their health, providing valuable insights and generating data for further research and targeted treatments.
Menstrual tracking apps, such as Clue and Flo, allow women to monitor their cycles, predict ovulation and manage symptoms, providing personalized health insights. Ovulation monitors like Ava and Mira enhance fertility tracking with real-time data, useful for those trying to conceive or avoid pregnancy.
Pelvic Floor training systems such as the Leva Pelvic Health System, Perifit deliver biofeedback and digital feedback to their companion smartphone apps to guide and monitor pelvic floor strengthening and coordination training for incontinence conditions. Other systems such as the Innovo and Elitone send neuromuscular stimulation to strengthen and re-educate the muscles of the pelvic floor. These systems can all be used at home.
Wearable devices like the Apple Watch and Fitbit track health metrics such as heart rate, sleep patterns and physical activity. These wearables now include features for women’s health, like cycle tracking and reminders, offering continuous health monitoring and comprehensive data for users and healthcare providers.
Diagnostic tools in FemTech include home testing kits for STIs and fertility, allowing women to gain health insights from home. Companies like Modern Fertility offer hormone testing kits that provide detailed information about fertility health, facilitating informed reproductive planning.
Telehealth platforms offer specialized care for women’s health issues through virtual consultations, improving access to care, especially in remote areas. Educational platforms provide resources on topics like menstrual health and menopause, empowering women with the knowledge to manage their health effectively.
FemTech’s impact is significant. It improves access to care, especially in underserved areas, provides personalized health insights and generates data to advance research. FemTech empowers women by giving them control over their health and the tools to manage it effectively.
The Role of Precision Medicine in Women’s Health
Precision medicine leverages genetic, molecular, lifestyle and environmental information to tailor treatments to individual patients. It moves away from a one-size-fits-all approach (Figure 2).
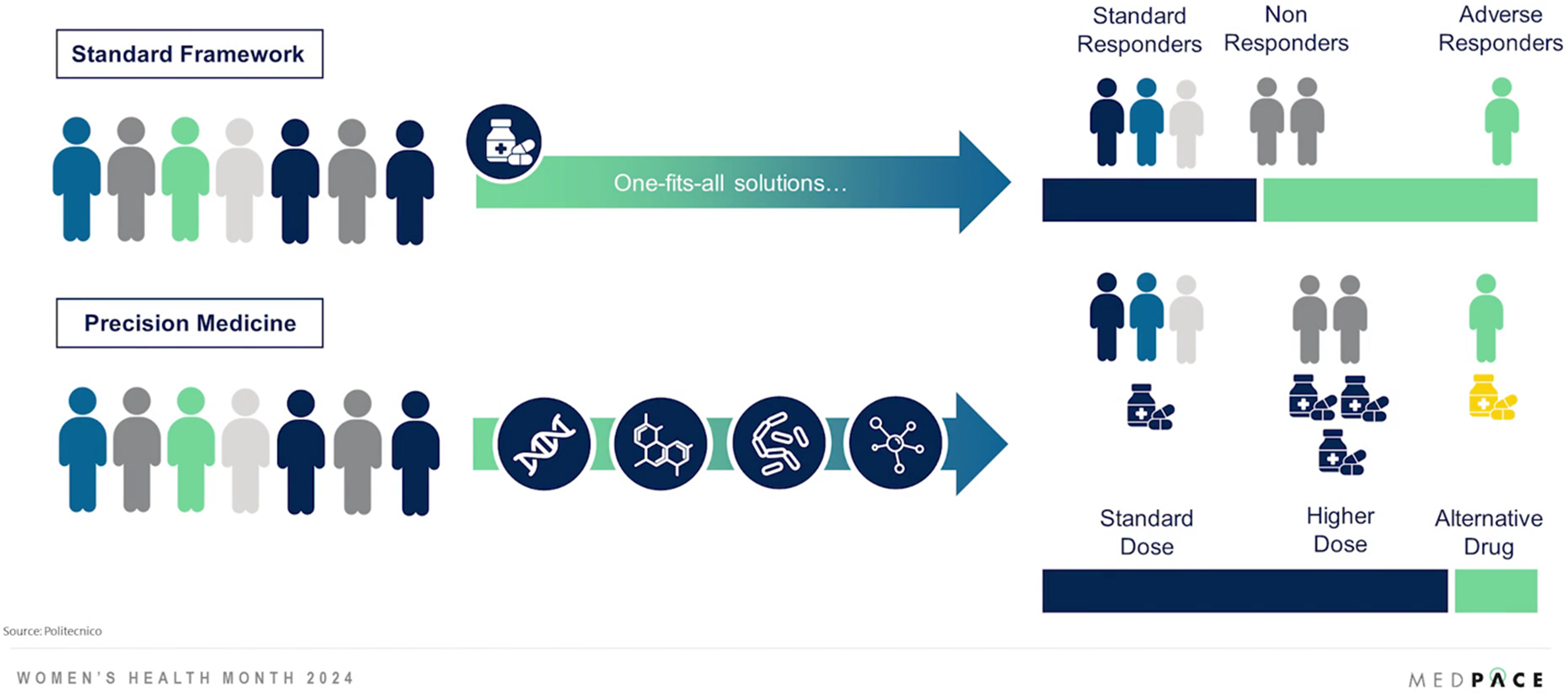
Figure 2. Precision medicine acknowledges the unique biological and genetic differences that affect disease manifestation and treatment response.
In oncology, precision medicine has already made significant progress by basing treatments on the genetic profiles of tumors. Precision medicine benefits breast cancer treatment. Identifying genetic mutations like BRCA1 and BRCA2 allows for personalized screening and preventive measures. Targeted therapies based on the genetic characteristics of tumors can improve survival rates and reduce treatment side effects.
This success is paving the way for its application in other areas of women’s health, particularly chronic conditions like endometriosis and menopause.
For instance, precision medicine can identify specific genetic and molecular markers associated with endometriosis, leading to more targeted and individualized therapies that address the underlying causes rather than just symptoms. This can enhance pain management and fertility outcomes, improving the quality of life for affected women.
Another promising area for precision medicine is menopause. Menopausal symptoms such as hot flashes, night sweats and mood changes vary widely. Precision medicine can tailor hormone replacement therapies and other treatments based on an individual’s genetic profile and lifestyle, ensuring more effective and safer management of symptoms.
Polycystic ovary syndrome (PCOS), affecting hormone levels, metabolism and overall health, can also benefit from precision medicine. Identifying specific genetic and hormonal imbalances in PCOS patients can enable more effective, individualized treatment plans, improving symptom management and fertility outcomes.
By tailoring treatments to individual genetic and molecular profiles, precision medicine can improve the effectiveness of therapies, reduce adverse effects and enhance overall patient outcomes.
Clinical Considerations for Women’s Health Clinical Development
Enhancing women’s health clinical trials involves several key strategies to ensure research success and address women’s unique needs.
A thorough analysis of the disease’s complexity, including pathophysiology, natural history and treatment landscape, is essential. This understanding allows for tailored study designs that capture meaningful endpoints and accurately reflect the disease’s impact on women’s health.
Inclusivity is crucial. Patient demographics such as age, ethnicity, comorbidities and socioeconomic status must be evaluated to ensure the diversity and generalizability of study results. Special efforts should be made to recruit and retain underrepresented groups, ensuring equitable access to healthcare outcomes.
“We aim to conduct research that generates robust evidence to advance healthcare for women worldwide.”
— Beth Tulip
Navigating regulatory requirements specific to women’s health research involves addressing gender-specific endpoints, safety considerations and ethical standards. Collaborating with investigators and regulatory authorities ensures compliance with local and international guidelines, fostering transparency and trust.
Evaluating potential study sites requires a rigorous assessment of infrastructure, including facilities, equipment and capabilities, to support the study protocol’s requirements. Patient access and recruitment potential, considering demographics, disease prevalence and willingness to participate, are also critical.
Sites with a strong track record in women’s health studies, particularly in recruitment and retention, adherence to protocols, data quality and regulatory compliance, should be prioritized to ensure successful study execution.
Embedding the medical leadership team in the clinical development process from proposal to study completion ensures consistent involvement and expertise. Collaboration with clinical operations teams allows for cross-therapeutic and cross-functional integration, resulting in optimal trial execution and improved outcomes.
Leveraging advanced technologies is vital. Using electronic patient-reported outcomes (ePRO), electronic clinical outcome assessments (eCOA) and electronic diaries (eDiaries) for data collection and management ensures compliance and facilitates real-time monitoring. The benefits of ePRO/eCOA/eDiaries are shown in Figure 3.
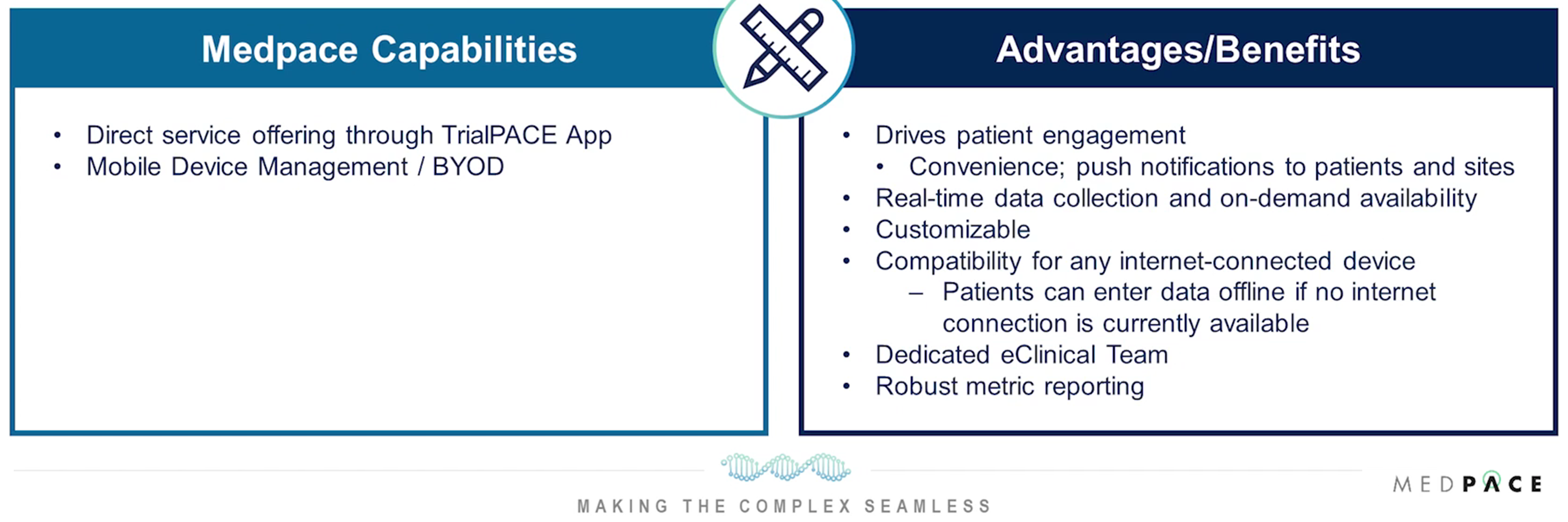
Figure 3. Medpace’s ClinTrak® ePRO/eCOA/eDiary allows patients to document and provide responses on health status, symptoms, etc., through an electronic device.
Decentralized trials with virtual components allow also participation from home, reducing barriers.
Addressing recruitment and retention challenges involves building trust between patients and physicians, developing standards based on lived and research experiences with marginalized communities, and addressing common barriers to participation.
Providing flexible trial hours, childcare, travel provisions, and personalized advertising can also help ensure high participation rates.
The evolving landscape of women’s health, as discussed by Dr. Ju, Dr. Shah, Beth Tulip and Dr. Jansen, presents numerous opportunities for innovation. Leveraging technological advancements and adopting comprehensive, inclusive clinical research strategies can improve women’s quality of life and address long-standing gaps in care.
To learn more about the recent advancements in women’s health clinical trials, visit Medpace’s Women’s Health webpage and contact their experts today.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.








Join or login to leave a comment
JOIN LOGIN