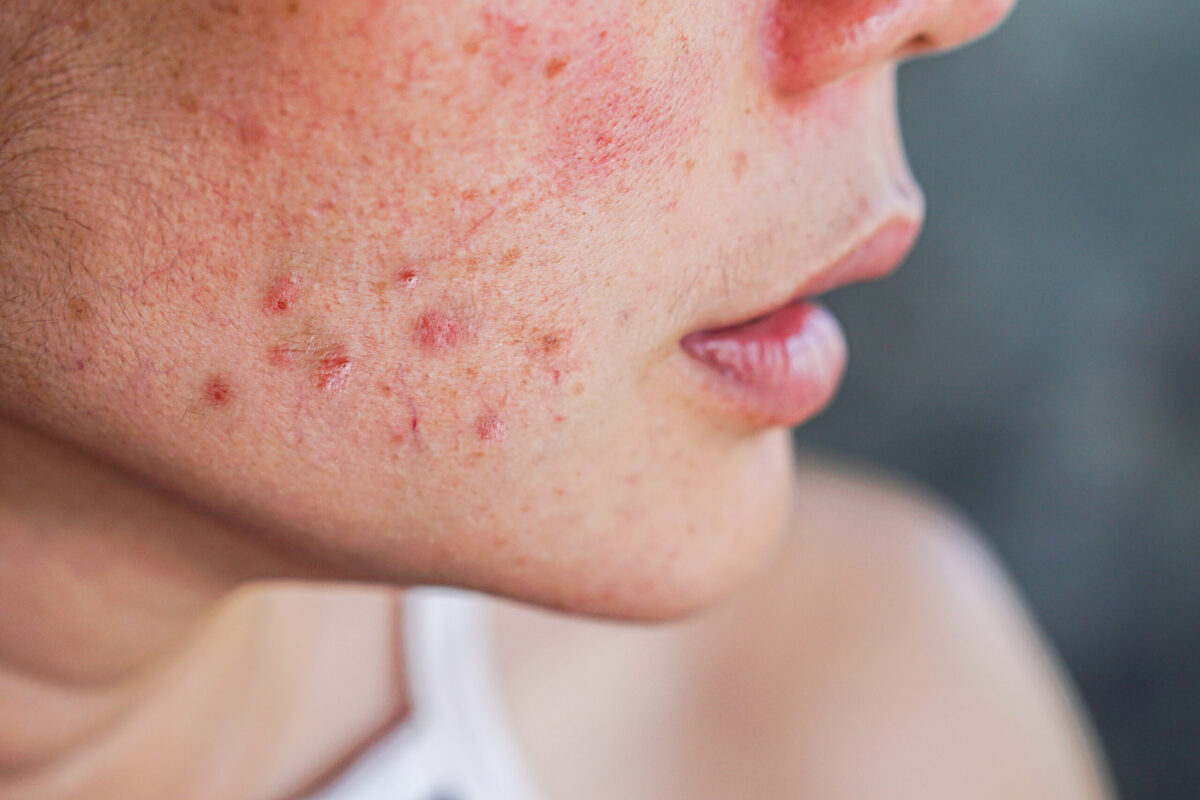
The US Food and Drug Administration (FDA) has approved not one, but two, new orally administered drugs for the treatment of moderate to severe eczema (atopic dermatitis), which include AbbVie’s Rinvoq (upadacitinib) and Pfizer’s Cibinqo (abrocitinib).
While Rinvoq was approved for eczema patients 12 years of age and older, Pfizer’s Cibinqo was approved for use in adults only. The treatments are indicated for patients in cases where they have not responded to previous eczema treatments, the condition is not well controlled with other medications and when the use of other drugs is not advised.
For AbbVie’s Rinvoq, the FDA approval is an expansion of its approval for the treatment of rheumatoid arthritis and psoriatic arthritis. On the other hand, this is the first US approval for Cibinqo.
Both Rinvoq and Cibinqo are Janus kinase (JAK) inhibitors that suppress inflammation by targeting specific JAKs in the JAK-STAT pathway. Both are also taken orally, which gives them an edge over Sanofi and Regeneron’s jointly developed biologic Dupixent (dupilumab) that is administered as a subcutaneous injection for the treatment of moderate to severe eczema in individuals six years of age and older. The drug is a monoclonal antibody that targets the IL-4 receptor alpha, which serves as a receptor for the closely related cytokines IL-4 and IL-13.
Dupixent has been a market hit since it was first approved by the FDA in 2017. It has been enjoying a stellar market run with global net sales having increased by 55 percent in the third quarter of 2021 to $1.66 billion compared to the third quarter of the previous year. It registered $4.4 billion in sales in the first three quarters of 2021.
Related: Pharma TV Ad Spending Trends: Companies in the Top Ten this Week
According to Pfizer, up to ten percent of adults and 20 percent of children around the world suffer from eczema, with a third of patients experiencing moderate to severe disease. In the US, there are around 6.5 million adults and 3.2 million children with moderate to severe eczema.
Rinvoq and other oral JAK inhibitors including Pfizer’s Xeljanz (tofacitinib) and Eli Lilly’s Olumiant (baricitinib) recently underwent a prolonged review by the FDA over safety concerns. Last month, the FDA finally granted Rinvoq expanded approval for psoriatic arthritis and Xeljanz for active ankylosing spondylitis; however, this approval came with strict warning labels that caution users about higher risks of serious heart issues, including major adverse cardiovascular events (MACE) like myocardial infarction and stroke, and cancers, such as non-melanoma skin cancer (NMSC) and lymphoma, compared to TNF blockers.
Rinvoq’s recent approval as an eczema treatment includes two dose strengths (15 mg and 30 mg). The approval was based on data from a Phase III trial that included over 2,500 patients evaluated across three different studies. In all of the studies, a significant improvement in itch relief was seen as early as the first week compared to placebo. AbbVie reported that Rinvoq (15 mg and 30 mg, once daily) monotherapy and as a combination therapy with topical corticosteroids met all primary and secondary endpoints at week 16, with some patients achieving higher levels of skin clearance.
Cibinqo’s approval was based on safety and efficacy data from three randomized, placebo-controlled Phase III clinical trials that included 1,615 patients in total and patients were12 years of age and older. The trials assessed measures of improvements in skin clearance, itch relief, disease extent and severity. One of the studies included an active control arm with dupilumab compared with placebo. Cibinqo met the co-primary endpoints of the trial at week 16, with significant improvements in the measured indicators including eczema severity observed with 100 mg and 200 mg doses.
AbbVie is also looking to benefit from Rinvoq because its best-selling drug Humira, which accounts for almost 40 percent of the company’s net revenue, is in for competition with biosimilars of the drug slated to begin hitting the market in 2023. AbbVie has been accused of creating a monopoly with Humira through price gouging and blocking biosimilar competition by leveraging patent loopholes.









Join or login to leave a comment
JOIN LOGIN