Avicenna.AI, a leading medical imaging AI company, has received US Food and Drug Administration (FDA) 510(k) clearance for two of its AI-based tools, CINA-iPE and CINA-ASPECTS, which are designed to enhance the diagnosis and assessment of pulmonary embolism and stroke severity, respectively, via CT scans.
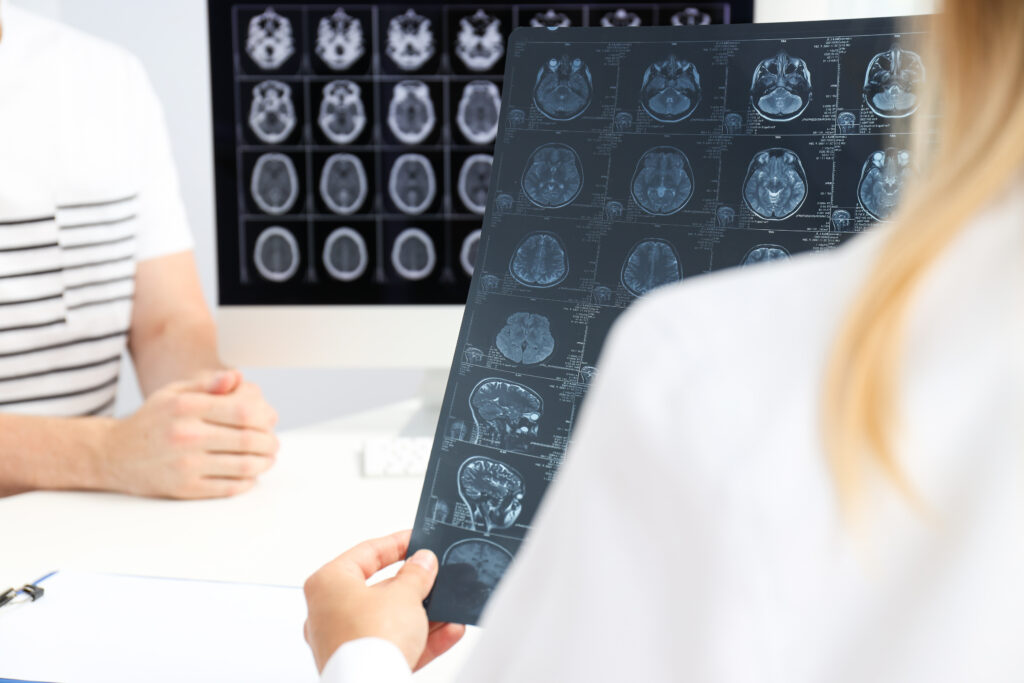
CINA-iPE was developed to help detect incidental pulmonary embolism during routine CT scans of other parts of the body. CINA-ASPECTS assists in the assessment of stroke severity by automatically processing non-contrast CT scans to calculate the ASPECT score — a method used to quantify brain damage severity following a stroke.
The France-based company says it is focused on leveraging a combination of deep learning and machine learning technologies to develop AI solutions that detect and prioritize life-threatening conditions within seconds, assess them for severity and notify clinicians.
CINA-iPE assists in the detection of pulmonary embolisms (blood clots in the lungs) which are often incidental findings during routine CT scans not initially intended to investigate for embolisms. The tool can be particularly useful in conditions like cancer where pulmonary embolism is a significant cause of mortality.
Among the greatest challenges in diagnostic imaging are delayed and missed findings. Avicenna.AI cited that while unsuspected pulmonary embolism is a common finding in routine CT scans of the chest, as little as 25 percent of emboli are reported during initial interpretation. This is particularly relevant in cancer patients, where pulmonary embolism is a significant cause of mortality.
Related: VUNO’s AI Tool for MRI Brain Scans Gets FDA Clearance
On the other hand, CINA-ASPECTS is the first FDA-cleared tool from Avicenna.AI in the computer-aided diagnosis (CADx) category, which the company says goes beyond identifying abnormalities in scans to provide an assessment of the severity of a condition.
CINA-ASPECTS automatically processes non-contrast CT scans of the brain and produces a score detailing stroke severity. The tool produces a heat map, infarcted regions and correct images from tilt.
The tool not only provides a topographic representation of affected brain regions but also enhances the reproducibility of stroke assessments, which can often vary depending on the radiologist’s experience.
“From day one, we have been committed to validating our AI tools on every type of CT scanner,” said Yasmina Chaibi, clinical affairs manager, Avicenna.AI, in a news release from the company announcing the FDA clearances. “In our validation studies, CINA-iPE achieved excellent sensitivity and specificity, demonstrating its ability to provide effective prioritization and triage on routine CT scans performed for other clinical indications than pulmonary embolism suspicion.”
Validated across 381 CT scans using 39 different scanner models from five leading manufacturers, Avicenna.AI’s pulmonary embolism detection tool CINA-iPE demonstrated high sensitivity and specificity in the validations, highlighting its effectiveness across various clinical settings.
Like CINA-iPE, CINA-ASPECTS was also rigorously validated, being tested on 200 scans from 27 scanner models, ensuring its robustness and reliability.
Both tools represent Avicenna.AI’s focus on developing tools that integrate AI within the radiology workflow, improving the speed and accuracy of medical diagnostics.
The company, which was founded in 2018, already has FDA clearance for its CINA-PE algorithm that is used for non-incidental findings of the disease.
CINA-PE and CINA-ASPECTS are additions to Avicenna.AI’s repertoire of other FDA-cleared, AI-based CT scan tools. These include tools for the automated detection of intracranial hemorrhage (CINA-ICH), aortic dissection (CINA-AD) and large vessel occlusion (CINA-LVO).
The company says all of its AI tools are seamlessly integrated into radiologists’ clinical workflow, automatically triggering and reporting algorithm results through the systems already used by radiologists.
According to GlobalData, the global AI in healthcare market was valued at $81.3 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of over 35 percent by 2030. AI in medical imaging is a large segment driving the market.
Current leaders in the CT imaging market include Philips, GE HealthCare and Siemens. According to GlobalData, Siemens and GE HealthCare lead with 33.5 percent and 24.2 percent, respectively, of the market share.
Philips and GE Healthcare are also investing in their own AI algorithms. In May 2023, Philips launched an AI-driven CT system that includes image-reconstruction and workflow-enhancing features. During the same month, GE HealthCare secured a $30 million order for its line of AI-powered CT scanners.
Avicenna.AI launched CINA-iPE in the US market in February 2023 and CINA-ASPECTS has also already debuted in the market.
If you want your company to be featured on Xtalks.com, please email [email protected].











Join or login to leave a comment
JOIN LOGIN