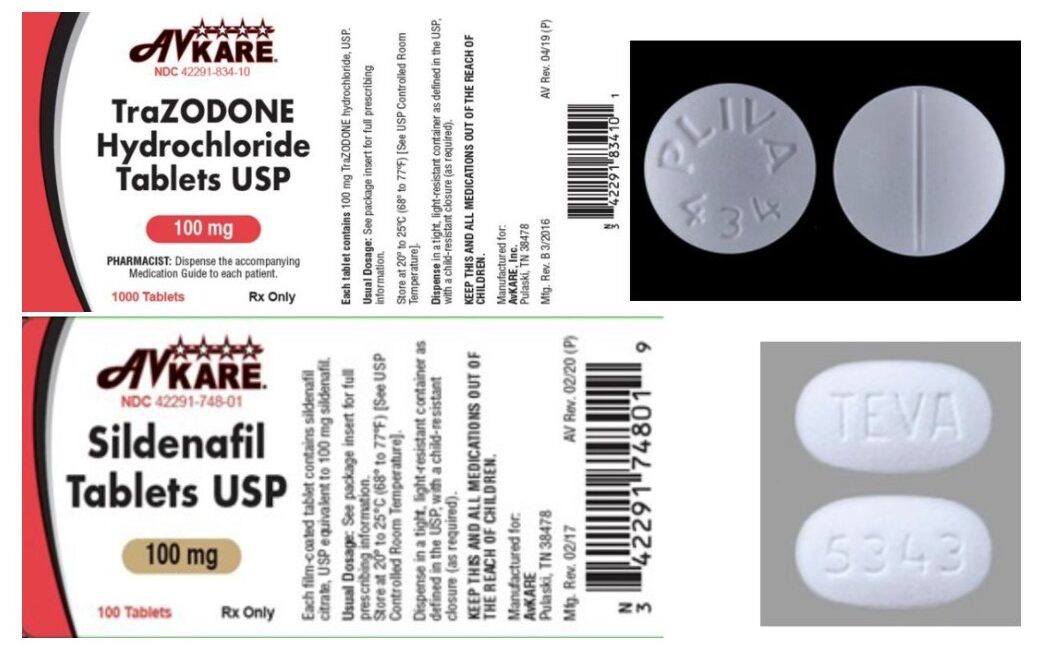
AvKARE, a generics pharmaceutical manufacturer based in Pulaski, Tennessee, has issued a recall on one lot each of its sildenafil 100 mg tablets and trazodone 100 mg tablets due to a packaging “mix-up” in which both drugs were filled into the same bottle. According to AvKARE, the error occurred at a third-party bottle filling facility. The identity of the facility was not disclosed.
When contacted by Xtalks, the company declined to comment on the recall.
The products affected by the recall are 100-count bottles of sildenafil (NDC 42291-748-01) with the lot number 36884 and a listed expiration date of 03/2022; and 1,000-count bottles of trazodone (NDC 42291-834-10) with the lot number 36783 and a listed expiration date of 06/2022. AvKARE said the mispackaged drugs were distributed nationwide.
While the company has yet to receive any adverse event reports as a result of the mispackaged tablets, unintentional consumption of the drugs has the potential to negatively affect a patient’s health. Silendafil — a generic form of the blockbuster erectile dysfunction drug Viagra — is contraindicated with nitrates, a class of prescription drugs commonly prescribed to patients with diabetes, high blood pressure and heart disease.
“Sildenafil may interact with nitrates found in some prescription drugs (such as nitroglycerin) lowering blood pressure to dangerous levels,” stated the recall notice posted by AvKARE.
The major depressive disorder treatment trazodone has a number of side effects — including drowsiness, dizziness, sedation and blurred vision — all of which are particularly concerning for elderly patients who are already at an increased risk of falls.
There are no known interactions between sildenafil, a phosphodiesterase-5 (PDE-5) inhibitor, and trazodone. However, Drugs.com lists 578 drugs that have a known interaction with trazodone, and 291 drugs that interact with sildenafil.
Interestingly, a 2007 article published in The Journal of Sexual Medicine investigated the efficacy of a combination therapy of sildenafil and trazodone in the treatment of erectile dysfunction in men who had previously failed to show a treatment response to sildenafil alone.
The small study included just 18 participants and showed mixed results with 12 of the men (66.7 percent) exhibiting a considerable improvement in sexual function, as evaluated by the Erectile Dysfunction Intensity Scale (EDIS).
On its own, trazodone has also been investigated as a potential treatment for erectile dysfunction; however, this drug has been implicated in 79 percent of reported cases of psychotropic-drug–induced priapism, a potentially-dangerous condition characterized by painful, prolonged erections.
Founded in 2007, AvKARE is a subsidiary of Amneal Pharmaceuticals, a global pharmaceutical manufacturer focused on the production of generics, specialty products and biosimilars. Amneal acquired AvKARE in December 2019, just one month after the company announced a voluntary nationwide recall of generic ranitidine due to potential contamination with N-nitrosodimethylamine (NDMA), an environmental contaminant and possible human carcinogen.
A recall announcement issued by pharmacy benefit manager Express Scripts listed AvKARE as the manufacturer of the affected lots of ranitidine. According to Drugs.com, AvKARE manufactures and distributes over 189 drugs in the US.











Join or login to leave a comment
JOIN LOGIN