Radiopharmaceutical therapy (RPT) uses isotopes that are conjugated to tumor-targeting agents (such as peptides, antibodies and novel small molecules). Radionuclide-ligand conjugates, including radionuclides that are conjugated to antibodies or peptides, have been studied extensively. While considered a relatively new class of cancer drugs, isotopes employed as radiation therapy is not a new concept. Due to recent developments in RPT manufacturing and recognition, RPT for solid tumors has emerged as an exciting anti-cancer modality. Recent developments include:
- Industry attention and investment in progress beyond pre-existing, widely tested platforms
- Discovery that tumor cell signaling pathways are not as easily, or persistently blocked than previously thought
- Expansion to non-malignant, benign targets. This has inspired an incentive for identifying RPT agents with new applications.
A major hallmark of RPT is the ability to deliver high doses of radiation directly to a cell target with minimal toxicity. This grants a number of advantages in the treatment of diseases like cancer, some of which include: the effective and specific targeting of tumor cells; radionuclides with high linear energy transfer (LET) can effectively kill radioresistant cells (i.e., hypoxic cells, or poorly differentiated cells that often exhibit therapeutic resistance); lower whole body absorbed dose; and many available forms are possible, such as free inorganic, conjugated to antibodies, and small biomolecules.
There are also disadvantages associated with implementing radionuclide therapy. These disadvantages include the fact that RPT distribution may be nonuniform and unpredictably dispersed. The biodistribution can be anticipated by pre-clinical and clinical dosimetry studies but is often not 100 percent predictable. There also still remains the risk of off-target binding and unwanted absorption into surrounding or distant tissues organs which results in potentially dangerous radiation toxicity.
“Radiation therapy has been used for over a hundred years and the role of radiation therapy has increased with time in treating cancer due to its predictable, well-established mechanism to eradicate cancer cells differentially,” said Dr. Jess Guarnaschelli, MD, Senior Medical Director, Medical Department, at the global clinical contract research organization (CRO) Medpace.
“We anticipate applications of radiation therapy will continue to expand over the next several decades, and thus we need improved ways to apply it, safely, on a global scale, and without excess toxicity to the patients we are trying to help,” added Dr. Guarnaschelli.
In a recent webinar, Dr. Guarnaschelli reviewed the medical considerations involved in radiopharmaceutical clinical trials. This webinar included a discussion of the clinical operations considerations for radiopharmaceutical trials from Deborah Hirscher, MBA, MS, RN, ARNP, FNP, Senior Director, Clinical Trial Management, Medpace and Dr. Stephanie Millin, PhD, Clinical Trial Manager, Medpace. The webinar also featured Dr. Daphnée Villoing, PhD, Dosimetry Specialist and Senior Project Manager, Medpace, who spoke about core lab considerations and James Thomas, BSc (Hons), MA, Senior Director, Regulatory Submissions, Medpace who shared regulatory considerations for radiopharmaceutical clinical trials.
Watch the free, on-demand webinar to gain valuable insights from a group of experts about implementing radiation dose limits in radiopharmaceutical clinical trials.
How does Radiation Cause Cell Death?
Radiation causes single and double strand breaks in DNA and radiation damage to cells can occur from a direct or indirect action of radiation on DNA molecules.
When radiation directly hits the DNA molecule, this disrupts the molecular structure of the DNA and this structural change to the DNA can lead to cell damage or apoptosis.
The indirect action of radiation involves radiation hitting water molecules and other organic molecules in the cell, which results in the production of free radicals. These free radicals react with DNA molecules and cause molecular structural damage to the DNA, that can result in the impairment of cell function or apoptosis.
Although cells have DNA repair mechanisms, the damage from direct or indirect action of radiation on DNA can persist, and this may lead to cell death or the development of secondary malignancies years later.
How does Radiopharmaceutical Therapy Work?
Radiopharmaceuticals can deliver radiation directly to tumors and specifically to cancer cells. This relatively new class of drug consist of a radioactive compound, a carrier (a targeting molecule that recognizes and specifically binds to the target on the cancer cell) and a linker that joins the radioactive compound to the carrier (Figure 1).
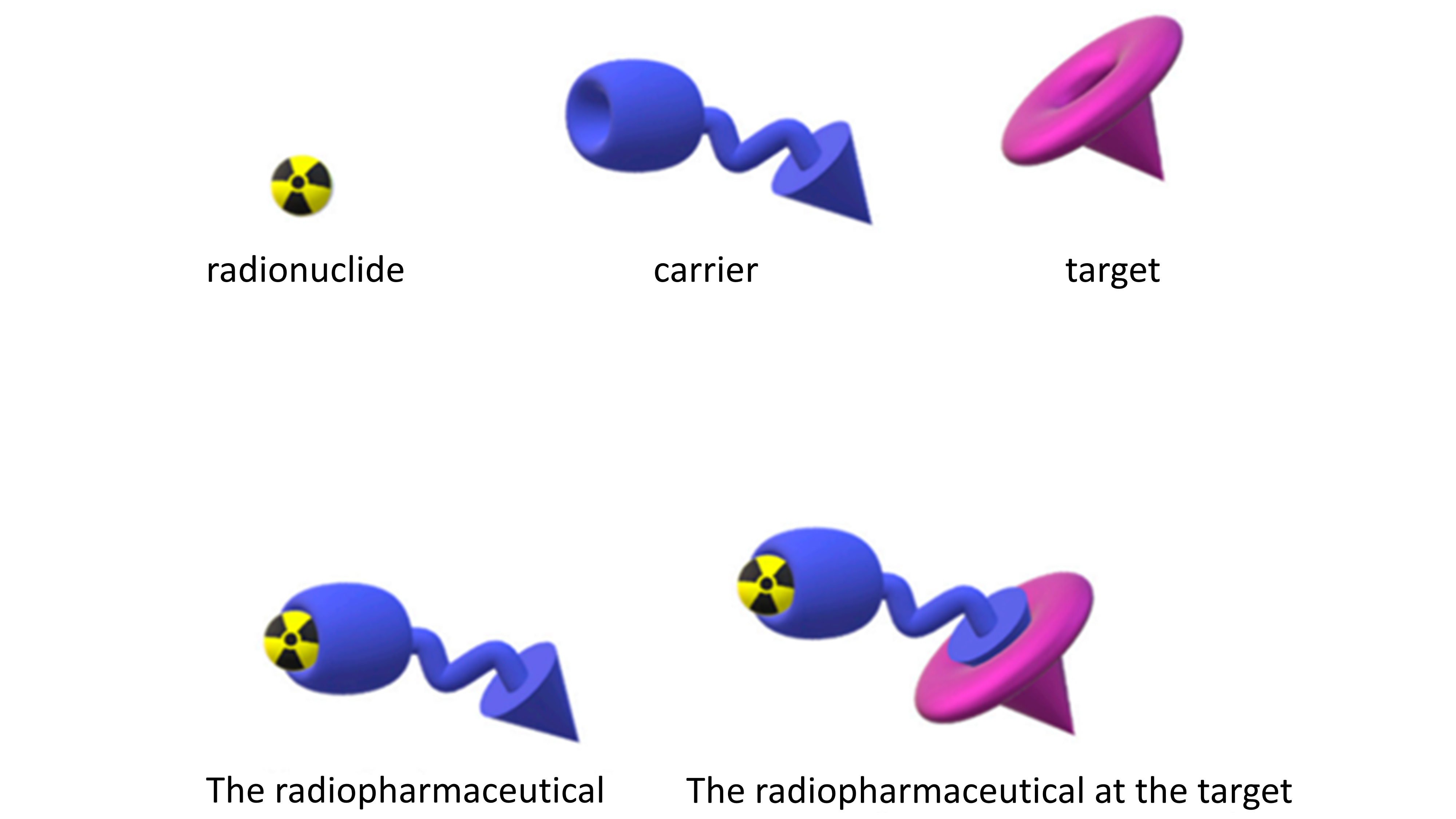
Figure 1. A depiction of the linkage of the radionuclide and the carrier to form the radiopharmaceutical that interacts with a specific biological target. Figure courtesy of Boschi et al., Appl. Sci. 2019, 9(12), 2526.
There are many radiopharmaceutical agents that are approved by the US Food and Drug Administration (FDA). Some of the most commonly used radiopharmaceutical therapies include 131I (radioiodine), 153Sm lexdronam (quadramet), 223RaCl2 (Xofigo) and [177Lu]LuDOTATATE, and their indication, type of particle emission and toxicities are shown in Table 1.
Table 1. Key approved radiopharmaceutical therapies and known toxicities.
| Product name | Indication | Type of particle emission | Toxicities |
|---|---|---|---|
| 131I (radioiodine) | Well differentiated thyroid cancer Toxic adenoma | Beta particle | Early: Xerostomia Nausea and vomiting Transient myelosuppression Radiation thyroiditis Nasolacrimal duct obstruction Transient thyrotoxicosis Late: Second malignancy Pulmonary fibrosis Hypothyroidism Permanent myelosuppression |
| 153Sm lexdronam (quadramet) | Painful multifocal osteoblastic skeletal metastases from prostate, breast, and lung cancer, and osteosarcoma | Beta particle | Myelosuppression Nausea and vomiting Constipation Asthenia Fever Anorexia Spinal cord compression Pain flare UTI |
| 223RaCl2 (Xofigo) | mCRPC with bone mets | Alpha particle | Bone pain Anemia Spinal cord compression Myelosuppression Constipation Nausea and vomiting Asthenia |
| [177Lu]LuDOTATATE | Adv. Midgut NET | Beta particle | Myelosuppression Nausea and vomiting Diarrhea Asthenia Anorexia Musculoskeletal pain Flushing Alopecia Myelodysplastic syndrome |
Radiopharmaceutical radionuclides emit alpha particles, beta particles or electrons. Similar to external beam radiation, each emission type from the radionuclides can damage the cell DNA resulting in cell death.
Therefore, given the potential for lethal cell damage, it is vital that radionuclides are directed to tumors and not to normal tissues. The biodistribution of the radiopharmaceutical agent must be known to anticipate tissue toxicity and side effects.
Medical Considerations for Radiopharmaceutical Trials
According to Dr. Guarnaschelli, it is important to consider organ dose constraints for patients enrolled into radiopharmaceutical and radiation oncology studies. The most typically thought of organs include the kidneys, liver, bladder, brain, heart, bowel, bone marrow, lung and spinal cord.
When delivering radiopharmaceuticals for therapy or external beam radiation therapy, it is crucial to take into account the agents that may impact radiation toxicity and a patient’s inherent sensitivity, for instance radiosensitizers, radioprotectors, sensitizing cytotoxic chemotherapy, targeted therapy/biologics and hyperthermia. Hereditary syndromes may also impact a patient’s inherent radiosensitivity, and some examples are shown below in Table 2.
Table 2. Examples of hereditary syndromes that impact radiosensitivity.
| Syndrome | Gene | Localization |
|---|---|---|
| Ataxia telangiectasia (AT) | ADA | 20q13.11 |
| Ataxia telangiectasia Like Disorder | ATLD | 11q21 |
| Nijemegen Breakage Syndrome | NBS1 | 8q21 |
| Fanconi Anemia FANCA FANCb FANCc FANCd FANCg | 16q24 - 9q22.3 3p26 9p13 |
|
| LiFraumeni Syndrome | CHK2 | 22q12 |
| RAD 50 | RAD50 | 5q31 |
| Breast Cancer | BRCA2 | 13q12 |
In addition, it is important to consider comorbidities when enrolling patients into a radiopharmaceutical trial as comorbidities may predispose a patient to increased radiation sensitivity and toxicity. Examples of comorbidities impacting radiosensitivity include scleroderma, systemic lupus erythematous, inflammatory bowel disease, HIV, diabetes/hypertension and multiple sclerosis.
Calculating Previous Radiation and Cumulative Radiation Exposure
Effective cancer treatment may need to combine external beam radiation therapy with radiopharmaceutical therapy, and this combination requires accurate three-dimensional dose calculations to avoid toxicity and evaluate efficacy, as well as real-time treatment planning.
“At Medpace, we encourage companies who are developing radiopharmaceutical trials to consider their enrolled patients’ previous and cumulative radiation exposure. Our in-house radiation oncology staff, dosimetry specialists and physicists work together to evaluate each enrolled patient’s past and clinical trial dosimetry,” said Dr. Guarnaschelli.
If a patient was previously treated with radiation prior to trial enrollment, Dr. Guarnaschelli recommends that the following be taken into consideration to decrease the chance of unexpected toxicity or surprising side effects:
- The site or target region and technique used in treatment
- The total radiation dose delivered, the number of fractions
- The previous modality for radiation treatment (e.g., photons, electrons and/or protons)
- A PDF of the treatment plan that has examples of other information including dose volume histograms (DVHs), digitally reconstructed radiographs (DRR), etc.
“Regarding record requests for patients with previous radiation, Medpace encourages sites to send a redacted, PDF of the patients’ previous radiation treatment plan to the center for evaluation,” said Dr. Guarnaschelli. “At Medpace, we have standard processes and procedures for collecting, evaluating, and incorporating previous radiation documents.”
If hypo-fractionated or stereotactic treatment was previously delivered, Dr. Guarnaschelli explained that the mean absorbed dose to the organs at risk do not necessarily correlate with tolerances from classic organ dose limits that are derived from historic external beam radiation therapy. Instead, the Equivalent Dose in 2Gy per fraction (EQD2) equation is used to convert hypo-fractionated and stereotactic dose regimens into a 2-Gy per fraction equivalent.
Clinical Operations Considerations for Radiopharmaceutical Trials
During the webinar, Ms. Hirscher, MBA, MS, RN, ARNP, FNP, Senior Director, Clinical Trial Management, Medpace, emphasized that every clinical trial team member plays a role in patient safety and that early detection of error is the best strategy to limit any consequence or complication from radiation exposure.
Anyone who touches the patient or reviews the paper or electronic Medical Record (MR) can note significant changes in patient status between visits. A clinical research coordinator (CRC), data coordinator or study nurse may be the first to note a potential MR note or exam finding that must be escalated to the investigator for immediate review.
Observations Related to Dosing
In the pre-screening process, it is important to collect all the information needed to assess a patient’s eligibility for a radiopharmaceutical trial. Ms. Hirscher explained that this includes collecting all the documentation for previous and cumulative radiation exposure calculation.
Clinical operations staff need to ensure that emergency equipment/medication is immediately available within the direct environment of radiation dosing. In addition, the principles of time, distance and shielding must be followed during dose administration to prevent unintended radiation exposure.
The drug administration site should be frequently assessed, particularly the peripheral lines, for signs of extravasation. “It is important to ensure that we have got a good plan in place, and we have that authorized user in the building available to come and assist as indicated,” said Ms. Hirscher.
In addition, it is important to review dosing documentation to ensure dose administered is within ±10 percent of the prescribed dose. According to Ms. Hirscher, anything over 10 percent must be immediately reported to the Medical Monitor/Sponsor and then the follow-up recommendations must be provided to the dosing team.
Assessing Adverse Events in Radiopharmaceutical Clinical Trials
It is important to use open-ended questions when talking to patients about their response to treatment to collect comprehensive events.
Also, any provider needs to recognize all described adverse events noted in the MR since the last patient study visit. In addition, any observations or complaints noted by the Nuclear Medicine staff during imaging studies will need to be paid attention to. Any new events should be compared to medical history and determined if worsening or not.
Any physical exam findings and lab results related to early radiation effects — such as abdominal discomfort, indicators of bone marrow suppression or findings related to the skin, mucosa and salivary glands — will need to be noted.
Targeted physical exam and lab assessments during safety follow-up visits can identify late radiation effects, and some of the late radiation effects can be seen from neurologic, pulmonary, renal and late bone marrow failure.
During the entire process of assessing adverse events, timely investigator assessment needs to be ensured to determine a relationship or lack thereof to radiation exposure.
“We need to make sure that the adverse event logs are completed by the coordinator so that the principal investigator can see the adverse event logs in real-time, and we can get that information into the database which everybody has access to,” said Ms. Hirscher. “I recommend establishing a routine weekly meeting with the investigator, and maybe only a five to 10-minute meeting is required.”
Therefore, adverse event data must be entered into electronic data capture (EDC) in real-time, so that data is available to Safety/Medical Monitors and the Sponsor. In addition, it is good practice to identify any trends to be shared proactively with the wider study team on safety calls.
Maintaining Oversight of Cumulative Safety Events
To gather a full view of the safety profile in a radiopharmaceutical trial, it is crucial to set up a study with processes in place to keep oversight of study-level, cumulative safety events and radiation toxicities.
“When we are setting the study up, we need to ensure that radiation related toxicities are identified in a timely manner and that the processes in place to identify and report them are as robust and as reliable as those that are already very well established for the more traditional DLTs [dose-limiting toxicities] and AEs [adverse events],” said Dr. Millin, PhD, Clinical Trial Manager, Medpace.
“This allows us to be confident in our identification of study triggers such as stopping rules or SRC [Safety Review Committee]/DSMB [Data and Safety Monitoring Board] milestones and that any escalation or de-escalation component of the study is being carried out correctly. That ultimately ensures the safety of our patients,” added Dr. Millin.
There are multiple formats and sources of possible safety data in a radiopharmaceutical trial, such as an electronic case report form (eCRF), safety database, central lab and dosimetry reports. The additional data sources and data flows from the calculation and reporting of data specific to radiation will need to be considered: for instance, the eCRF may need to be adapted to allow for accurate reporting of events from radiation exposure.
In addition, data from different sources and formats must be put together in an informative way for team members specialized in radiation, such as dosimetry specialists, and those not specialized in radiation as well.
Once the processes involved in safety oversight are set up and robust, any toxicity identified will be flagged to the relevant study team members. Additionally, the emerging safety profile can be comprehensively reviewed to identify any actions that may be needed at a study level.
Setting Up for Dosimetry from An Operational Perspective
During the webinar, Dr. Millin discussed the operational considerations for setting up for dosimetry in radiopharmaceutical clinical trials. There are numerous considerations for data requirements, timelines and process development that are all interconnected and that need to be incorporated into the final operational strategy for setting up dosimetry (Figure 2).
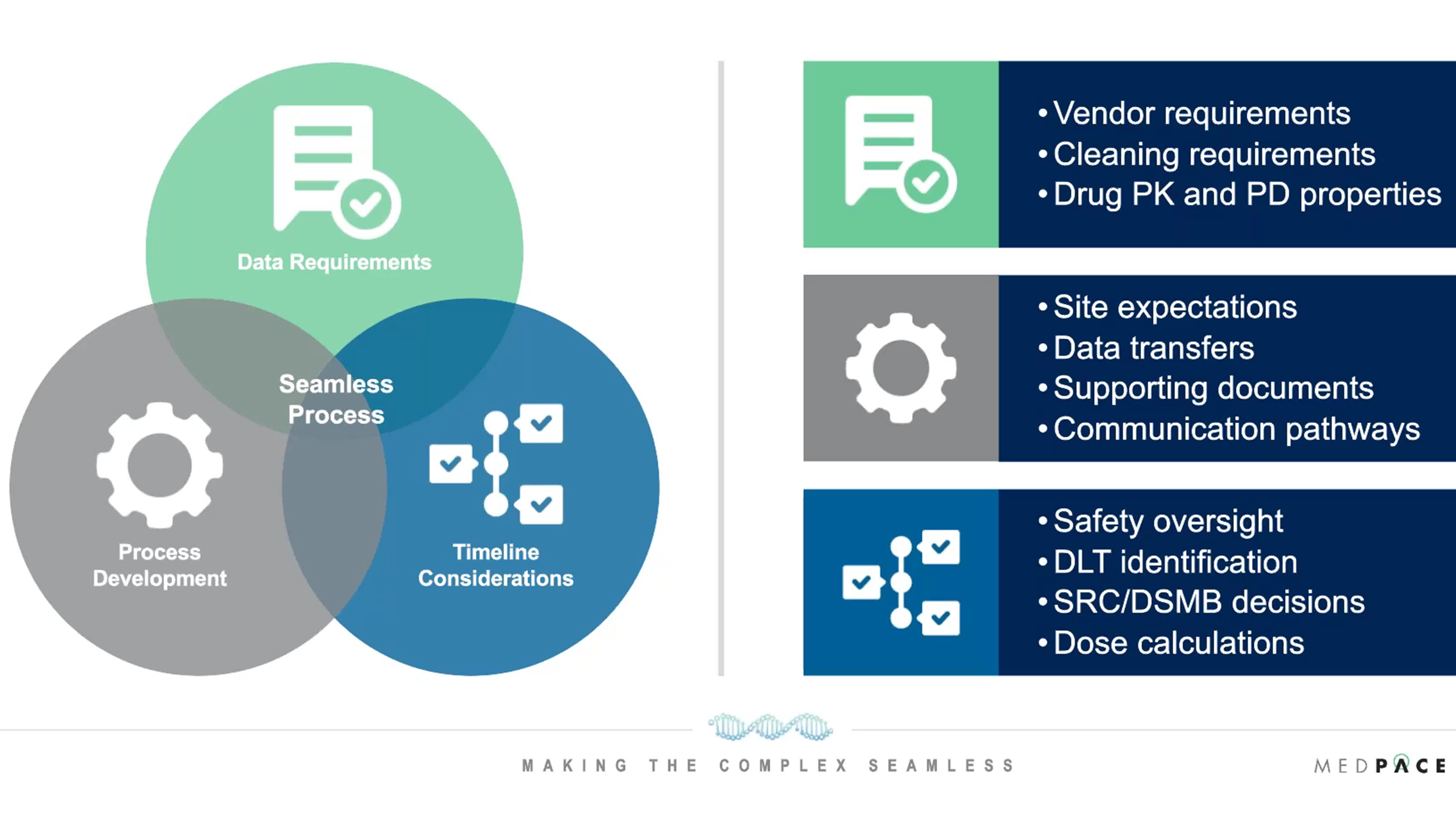
Figure 2. Operational considerations for setting up dosimetry in radiopharmaceutical clinical trials.
Regarding the data requirements for setting up dosimetry, it is important to consider what data points need to be collected from patients. Different dosimetry modeling may require different inputs and could involve phantom scans, calibration data, different imaging, qualification data, blood and urine data and more.
It is also necessary to assess the quality of the data and the level of cleaning and verification that will be required before dosimetry calculation starts. Dosimetry specialists need to be engaged early on to ensure they can work with the schedule of events that are planned in the study protocol, as dosimetry calculations can be very lengthy.
Timeline considerations must be given for safety oversight, DLT identification, SRC/DSMB decisions and dose calculations. For example, Dr. Millin discussed how it is necessary to recognize when a SRC meeting will occur, and how long attendees need to review the data ahead of the meeting to build processes able to meet those requirements.
A good process development strategy for setting up dosimetry evaluates site expectations and communication pathways to the site. In addition, all supporting documents for dosimetry need to be in place and data should be transferred efficiently, which requires that data is entered within a particular time frame.
“Dosimetry processes need to be considered in detail from the start of the study and all parties need to be engaged early on to ensure that they are seamlessly integrated and embedded into the study execution,” explained Dr. Millin.
Core Labs Considerations: Radiation Dosimetry Concepts
It is important to consider the dose limits in radiopharmaceutical clinical trials. In the dosimetry of radiopharmaceuticals, the absorbed dose is calculated by following a specific formalism developed by the Medical Internal Radiation Dosimetry (MIRD) Committee.
The MIRD-developed equation for calculating the absorbed dose to a target region is shown in Figure 3. Dr. Villoing, PhD, Dosimetry Specialist and Senior Project Manager, Medpace Core Labs, explained how this absorbed dose equation is the product of the time-integrated activity in the source region (this corresponds to the total number of disintegrations that happened in that region of interest, which can be an organ or a tumor) with the S value, i.e. the absorbed dose in a target region per disintegration in source regions.
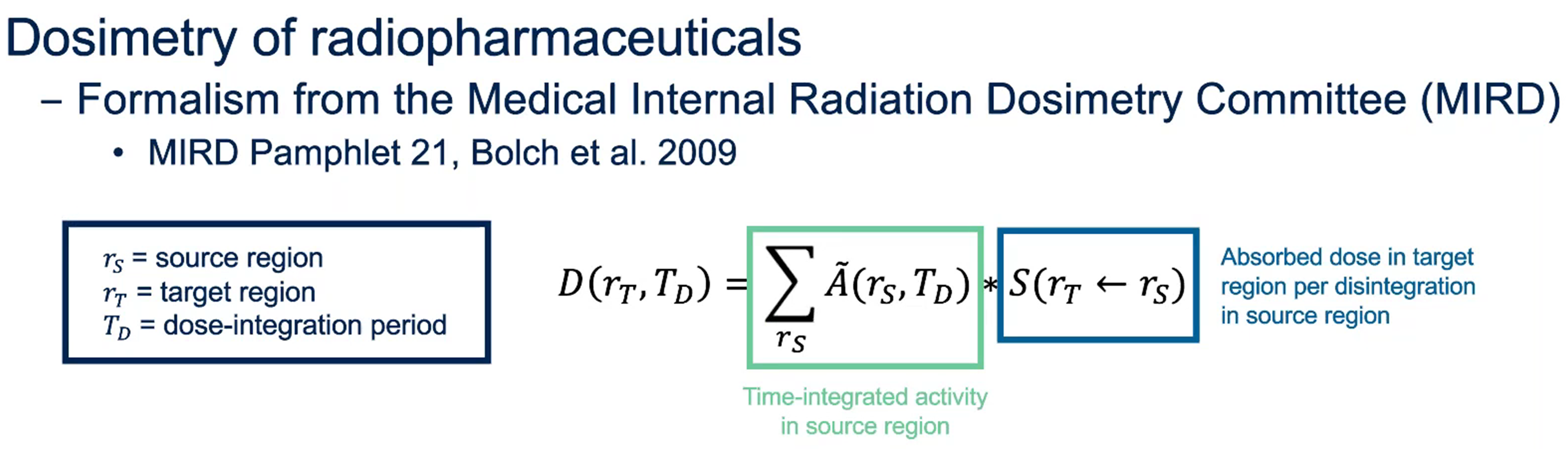
Figure 3. Basic concepts of dosimetry for radiopharmaceuticals: the frequently-used equation to calculate the absorbed dose D(rT,TD) to a target region.
When the source regions are also the target regions, energy deposition from the emitted particles is local and self-irradiation occurs in these organs or tissues.
When the source and target regions are different, cross-irradiation occurs and the target regions receive the energy of particles that were emitted somewhere else.
Model-Based versus Patient-Specific Dosimetry
One consideration in internal radionuclide radiation dosimetry is whether to do the absorbed dose calculations within the patient images (patient-specific dosimetry) or use anthropomorphic models that are representative of the human body (Figure 4).
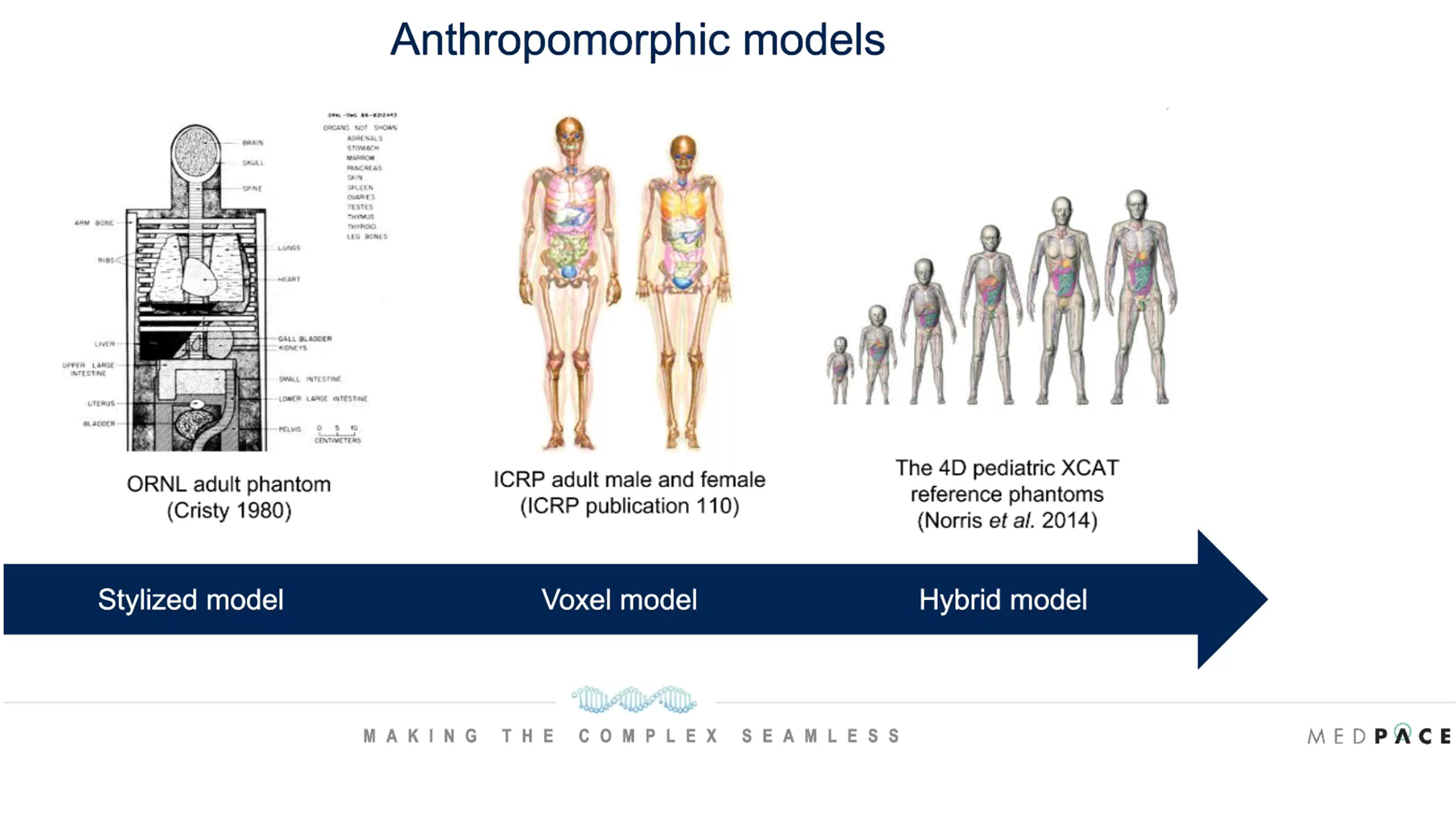
Figure 4. Anthropomorphic models used for internal dosimetry.
The development of anthropomorphic models began with stylized models in the 1980s, which were not very realistic of the human body. Later voxel models were used for internal dosimetry and now hybrid models are being used which are much more representative of the human body.
“It is recommended to perform patient-specific dosimetry for therapeutic compounds rather than model-based dosimetry since it accounts for intra-patient anatomical variation and way better tumor dosimetry,” said Dr. Villoing. “But patient-specific dosimetry involves complex in-house developments for applications in clinical trials, especially for emerging radionuclides. Therefore, model-based dosimetry is sometimes the most appropriate option.”
About dose limits, Dr. Villoing reminds that existing dose limits were established from external radiotherapy, which is a limitation for their application to radiopharmaceutical therapy.
Explaining Radiation Exposure in Informed Consent Forms
Dr. Villoing explained how informed consent forms sometimes refer to the effective radiation dose and this term should be used with caution because, as stated by Fisher and Fahey in 2018 in Health Physics, it is the “absorbed dose that is the relevant quantity for evaluating radiation dose to organs and tissues from exposure to external radiation and internal radionuclides in the workplace, the environment and in medical applications.”
The following inappropriate uses of the effective dose were reviewed during the webinar:
- To predict cancer risk among exposed persons
- In retrospective investigations of individual exposure and risk
- In treatment decisions
- To assess the radiation dose to medical patients.
Regarding the use of the effective dose in the informed consent forms of radiopharmaceutical trials, Dr. Villoing recommends considering the following options:
- If the situation involves therapeutic levels of activity: The use of effective dose and a comparison to natural background exposure is not appropriate. Absorbed doses to individual organs should be discussed. Specific language should be developed.
- If the situation does not involve therapeutic levels of activity: Risks can be considered “minimal” or “acceptable”. The use of effective dose and a comparison to natural background exposure may or may not be appropriate. Absorbed doses to individual organs may have to be provided. A proper evaluation needs to be made.
According to Dr. Villoing, Medpace can help with the evaluation of the consent language in radiopharmaceutical clinical trials.
Regulatory Considerations for Radiopharmaceutical Trials
James Thomas, BSc (Hons), MA, Medpace’s Senior Director of Regulatory Submissions, encourages that any information that is presented to regulators, ethical committees, review bodies or participants should display the technical aspects of the study in an accessible and engaging manner for the audience (Figure 5).
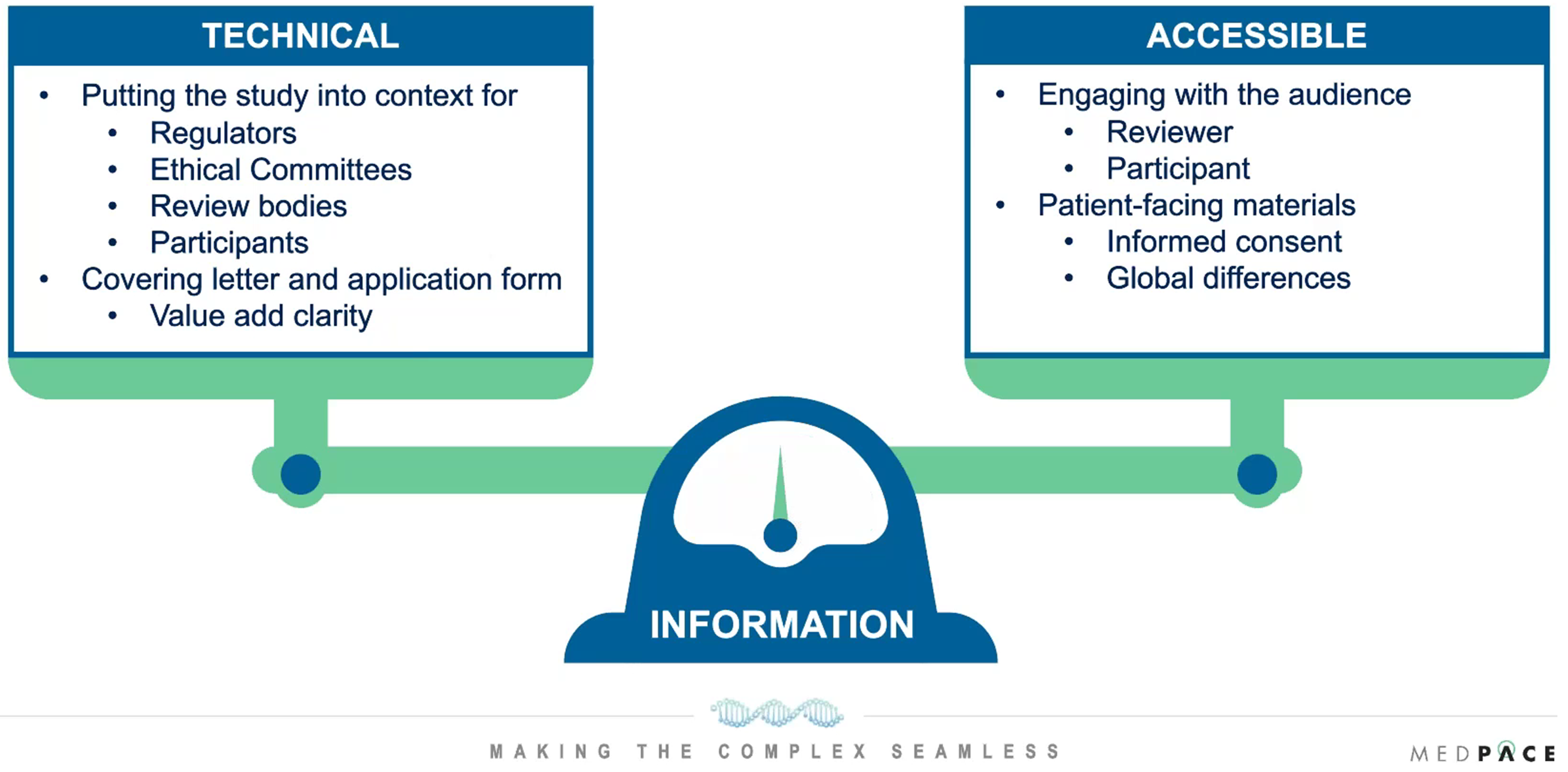
Figure 5. Information needs to be presented with the right balance of technical information and accessible information while keeping the audience in mind.
“It is about having the ability to have the right information to present to regulators, ethics committees, dedicated radiopharmaceutical review bodies who may have their own perspectives compared to participants who may look at it from a very different angle,” said Mr. Thomas.
Considerations for Patient-Facing Materials
Informed consent forms of radiopharmaceutical trials need to include risk language that explains the potential impact upon the participant’s body, such as immediate side effects and lifetime cancer incidence risk.
Precautions and safety measures may be covered in informed consent forms. For instance, study participants are usually told to avoid contact with pregnant individuals and small children less than 10 years old for several days after dosing, to shower at least once daily and to avoid close contact with their partners, family and friends.
Certain countries may also mandate specific wording to be used in the consent forms of radiopharmaceutical trials.
It is crucial to have education materials for patients and family about handling radioactive waste from saliva, sweat, blood and urine.
A special notification card may also be considered as subjects may set off radiation detectors at airports.
According to Thomas, a separate precautions document may be used to explain the participation risks in more detail, and it can be a useful reference for participants throughout the trial. The precautions document is also especially useful with repeat dosing where subjects may need to refer to precautions repeatedly over weeks and months.
“Our regulatory submissions team at Medpace cover the regulatory authorities and the ethics committees, which allows us to be able to understand the two different perspectives and it allows the right information to be presented in the right way,” said Mr. Thomas.\
To learn more about overcoming challenges associated with complex radiopharmaceutical clinical trials, watch Medpace’s on-demand webinar.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.



Join or login to leave a comment
JOIN LOGIN