Clinical research experts from the CRO Medpace shared insights about the operational and regulatory considerations for neuroscience trials with direct CNS administration.
According to the United Nations, up to one billion people — or nearly one in six people in the world — suffer from neurological disorders, from brain injuries and neuroinfections, to Alzheimer’s and Parkinson’s disease. According to an analysis of the 2013 Medical Expenditure Panel Survey, one in six US adults reported taking a psychiatric drug at least once in 2013. Global sales of over-the-counter and prescription products related to central nervous system (CNS) disease added up to $86 billion in 2019.
CNS drugs treat a range of neurologic and psychiatric disorders, such as psychosis, depression, Parkinson’s disease, multiple sclerosis and epilepsy.
One of the biggest challenges with developing therapies to treat CNS disorders is the delivery of systemically administered investigational products (IPs) to the brain, which is limited by the blood-brain barrier.
Direct CNS administration techniques facilitate drug delivery to the brain to treat neurological disorders and these approaches are vital for CNS targets. Some methods of direct CNS administration include intrathecal, intraparenchymal and intracerebroventricular (ICV) delivery.
“If you’re working on trying to make small molecules that get in and hit a target [in the CNS], widen your perspective. Think about all the other potential ways of directly targeting parts of the CNS and the targets in the CNS that you want in order to achieve your therapeutic goals for the patient,” says Dr. James Vornov, MD, PhD, Vice President of the Medical Department at the global clinical research organization (CRO) Medpace.
In a recent webinar, Dr. Vornov described the history and current progress in targeted CNS therapeutic delivery. In the same webinar, Dr. Filipe Rodrigues, MD/MSc, Medical Director at Medpace spoke about the modern-day applications of direct CNS administration, including intrathecal, intraparenchymal and ICV delivery. The webinar also featured Kelsey Carter, MS, Clinical Trial Manager at Medpace who discussed the site, operational and regulatory challenges for neuroscience trials with direct CNS administration along with the strategies to mitigate these challenges.
Watch the on-demand webinar to gain important insights from a team of experts about the modern-day applications and considerations of direct CNS administration in neuroscience trials.
Why is it Difficult to Develop CNS Therapeutics?
CNS drugs take more than a year longer to develop and are less than half as likely to receive marketing approval compared to other drugs, according to a 2014 study by the Tufts Center for the Study of Drug Development.
It is difficult to develop CNS drugs because the brain is a complex organ and there is a limited understanding of the mechanisms that underlie brain disease and how they are correlated to behaviour, cognition and other clinical symptoms. In addition, many CNS diseases are idiopathic diseases without links to causative agents or pathology.
“There are loose correlations between the pathology going on in the brain and clinical symptoms in neurology and psychiatry,” says Dr. Vornov. “We know that a lot of different pathologies, especially as we’ve entered the era of molecular medicine and gene therapies, cause many of the same symptoms and work through final common pathways. And so, we’ve generally tended to focus either at the symptomatic side or on trying to find common pathways to treat diseases.”
Also, traditional preclinical models may not accurately reflect the possible efficacy of compounds moving into Phase I trials, particularly with compounds that have new mechanisms of action.
A major obstacle for CNS drug developers is the inability to target therapies to the CNS broadly (for instance, a therapy that is orally bioavailable as a pill and has difficulty crossing the blood-brain barrier) and certain CNS regions more specifically. “Sometimes we can engage with the target that we’re interested in, but very often these drugs get through the entire brain. We get off-target pharmacology that makes the drug not tolerable or not very therapeutically useful,” says Dr. Vornov.
Targeted CNS Delivery: A Perspective on Where We’re Going
Direct local administration of drugs to the CNS is not a new concept and has been around since at least 1885 when cocaine was first administered for anesthesia through perforation of the subarachnoid space by a lumbar puncture.
The acquired knowledge of direct CNS administration techniques is growing, and these approaches are valuable for CNS targets.
During the webinar, Dr. Vornov discussed a few developments in targeted CNS delivery, which are summarized below.
Intrathecal Drug Systems are an Important Pain Treatment Modality
Intrathecal drug delivery involves an injection into the subarachnoid space of the spinal cord and there are programmable, implantable intrathecal drug infusion systems for the management of chronic pain (Figure 1). In 1982, the Infusaid pump by Shiley Infusaid Inc. was the first implantable continuous infusion pump for intrathecal delivery of drugs to treat chronic pain that was commercialized in the US. After that, the first implantable programmable pump for intrathecal delivery was approved by the US Food and Drug Administration (FDA) in 1991 and it was Medtronic’s SynchroMed pump.
Currently, the FDA only approves monotherapies of ziconotide, morphine and baclofen for intrathecal delivery.
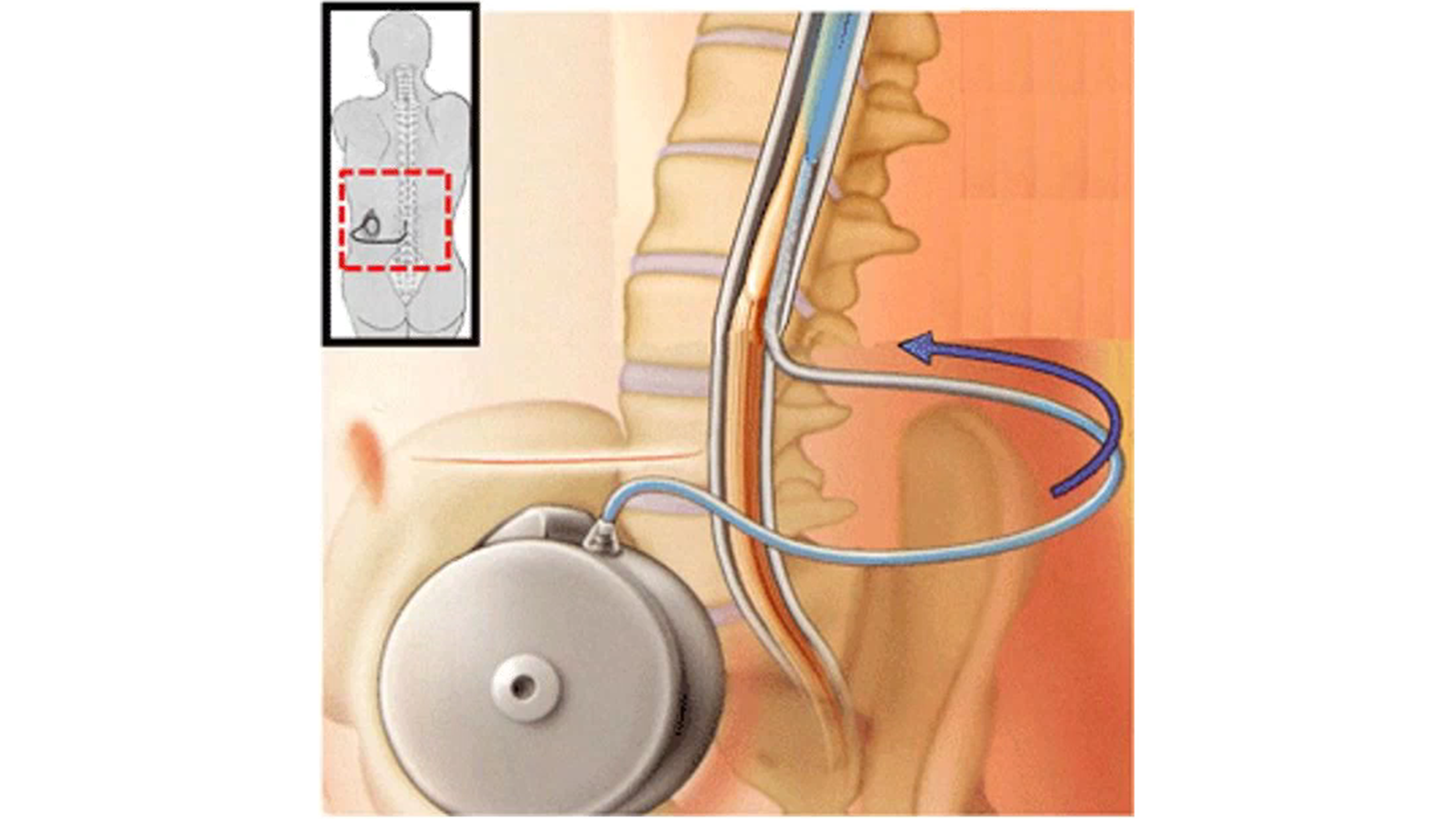
Figure 1. Intrathecal drug infusion systems are an important way of treating chronic pain.
An advantage of intrathecal delivery is that direct injection into the spinal canal may reduce systemic concentrations of the drug and allow for high target concentrations. For example, intrathecal injection of opioids that is 1/300 of the oral administration amount can result in the same pain control effect, and so the complications from opioid use may be minimized with intrathecal delivery.
Another advantage of intrathecal delivery is that it may allow therapies to gain access to the spinal space which may not have systemic access to the CNS. Such therapies may involve small interfering RNA (siRNA) or oligonucleotides in viral transfection platforms, large molecules and antibodies.
However, according to Dr. Vornov, the use of intrathecal delivery in drug development is unexplored for small molecules and peptides besides what has already been proved.
A disadvantage of using intrathecal drug delivery systems is that complications may occur, which include but are not limited to: drug delivery device-related complications from malfunction of the catheter or pump; magnetic resonance imaging (MRI)-related complications; human factors such as refill schedule mistakes, programming errors and incorrect refill and access port procedures; and drug overdose. Therefore, healthcare professionals should have a lot of experience with intrathecal drug delivery, be aware of the potential complications and know how to manage them if they arise.
Intrathecal Chemotherapy
Intrathecal chemotherapy is primarily used for leptomeningeal carcinomatosis, a cancer involving the arachnoid mater and pia mater.
There are two ways to inject anticancer drugs into the intrathecal space that holds the cerebrospinal fluid (CSF). One way is to inject the drugs into the lower section of the spinal column and the second way is to inject the drugs into the Ommaya reservoir, which was invented in 1963 by Ayub Ommaya. The Ommaya reservoir is a soft, dome-shaped device about the size of a quarter that is placed under the scalp during surgery, and it holds the drugs as they flow through a catheter into the ventricles of the brain (Figure 2).
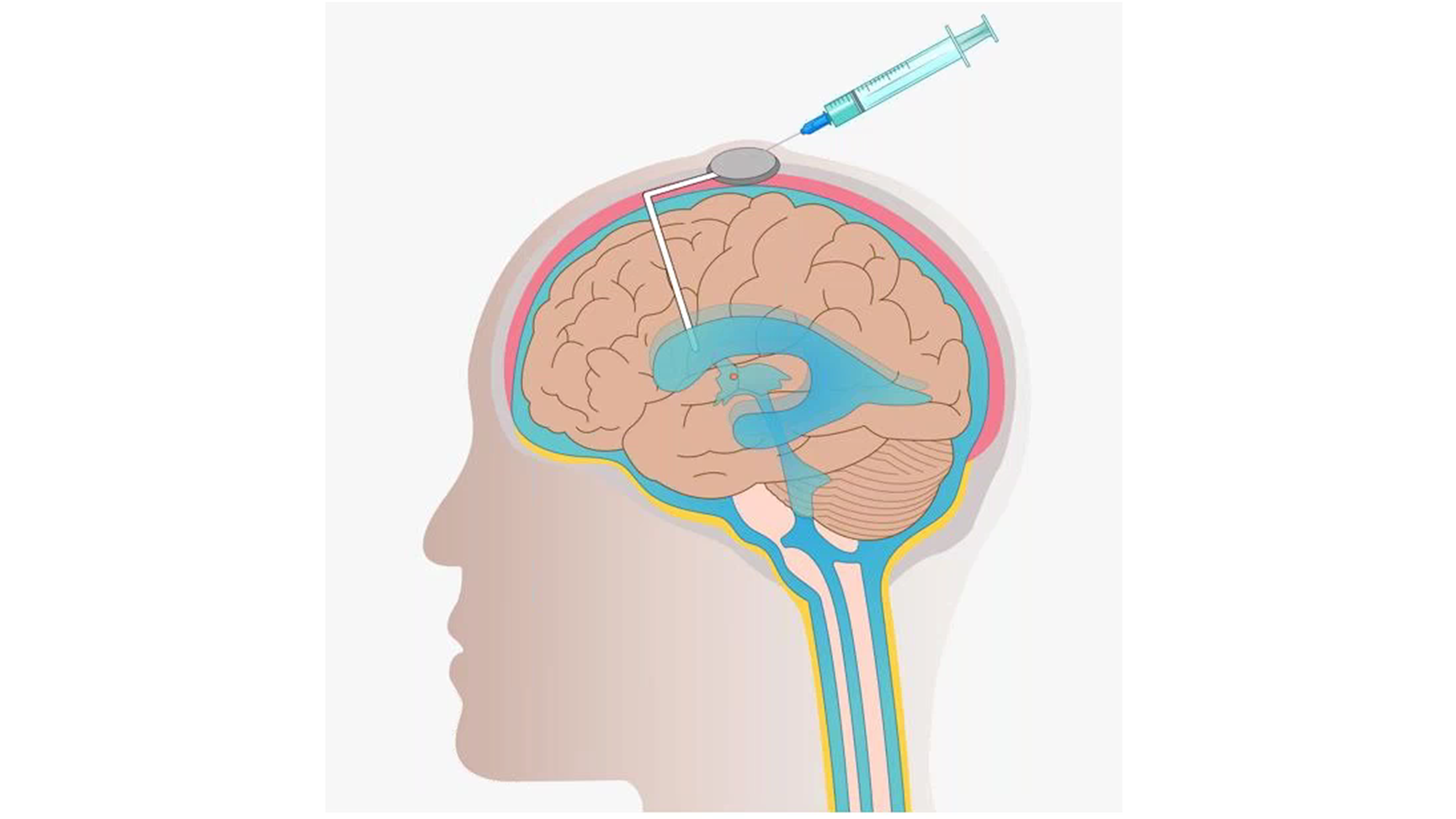
Figure 2. Intrathecal chemotherapy using the Ommaya reservoir to inject anticancer drugs.
According to Dr. Vornov, the potential of intrathecal chemotherapy for drug delivery is largely unexplored.
Dr. Vornov explained that drug stability is an issue when using a reservoir system because it is set up against the body and at room temperature. Therefore, it is better to use drugs that are stable at room temperature when using a reservoir system for intrathecal delivery.
“It’s much more difficult than a drug in a bottle or a pill that you can do standard stability testing on, knowing what the stability of your drug is. It’s interesting that what we have for use in this way are all known drugs that are being delivered through novel routes, rather than new chemical entities where we have many more regulatory hurdles to overcome,” says Dr. Vornov.
Nusinersen: A Landmark Therapy
Biogen’s nusinersen (brand name Spinraza) is an antisense oligonucleotide (ASO) therapy that is administered directly into the CNS by intrathecal injection through lumbar puncture.
Nusinersen was the first FDA-approved treatment for spinal muscular atrophy (SMA), the first approved nucleic-acid therapeutic for a neurologic disease and the first approved drug to correct defective splicing. More success follows nusinersen as it was also the first disease modifying drug for a neurodegenerative disease and the first drug to demonstrate prevention of onset in pre-symptomatic patients.
Nusinersen was truly a landmark therapy for neurological and neurodegenerative disease and according to Dr. Vornov, nusinersen is driving much of what is being currently seen in drug development.
What About Small Proteins and Antibodies Reaching Extracellular CNS Targets?
According to Dr. Vornov, there is plenty of evidence that at least some therapeutic antibodies can reach extracellular CNS targets. For instance, in 2021 the FDA granted accelerated approval to Biogen’s aducanumab for patients with Alzheimer’s disease with mild dementia or mild cognitive impairment stage of the disease. The accelerated approval was based on data from clinical trials that showed aducanumab can reduce brain amyloid beta plaques, which are biomarkers in Alzheimer’s disease that are likely to predict clinical benefit.
But according to Dr. Vornov, intracellular targets cannot be targeted using antibodies, and systemically administering antibodies to get into the CNS has not been explored that much outside of Alzheimer’s disease and amyloid as a target.
“Just for small peptides, we’ve had tons of programs and no peptide administered intravenously has ever been approved for a CNS indication despite many failed trials,” says Dr. Vornov. “It’s just not clear that that’s a viable method, but of course, direct CNS administration could be a completely different story, but it is something that has not been looked at in much extent.”
Advances in Stereotactic Technique
Stereotactic (or stereotaxic) techniques are used to permeate the blood-brain barrier to enhance drug delivery and this method offers many possibilities to target therapies. Stereotactic injection involves computer use and a 3D scanning device to inject drugs to specific regions of the brain.
There has been much progress since the first human stereotactic frame in 1918 to modern-day stereotaxic guidance systems such as the ClearPoint System by ClearPoint Neuro, Inc. (Figure 3). ClearPoint Neuro is a platform neurosurgery company focused on developing solutions for direct CNS delivery of therapeutics in preclinical studies and clinical trials across the world. An example of ClearPoint Neuro’s success in this endeavour is their SmartFlow Cannula that received FDA clearance for aspiration of CSF or injection of the chemotherapy drug cytarabine into the ventricle.
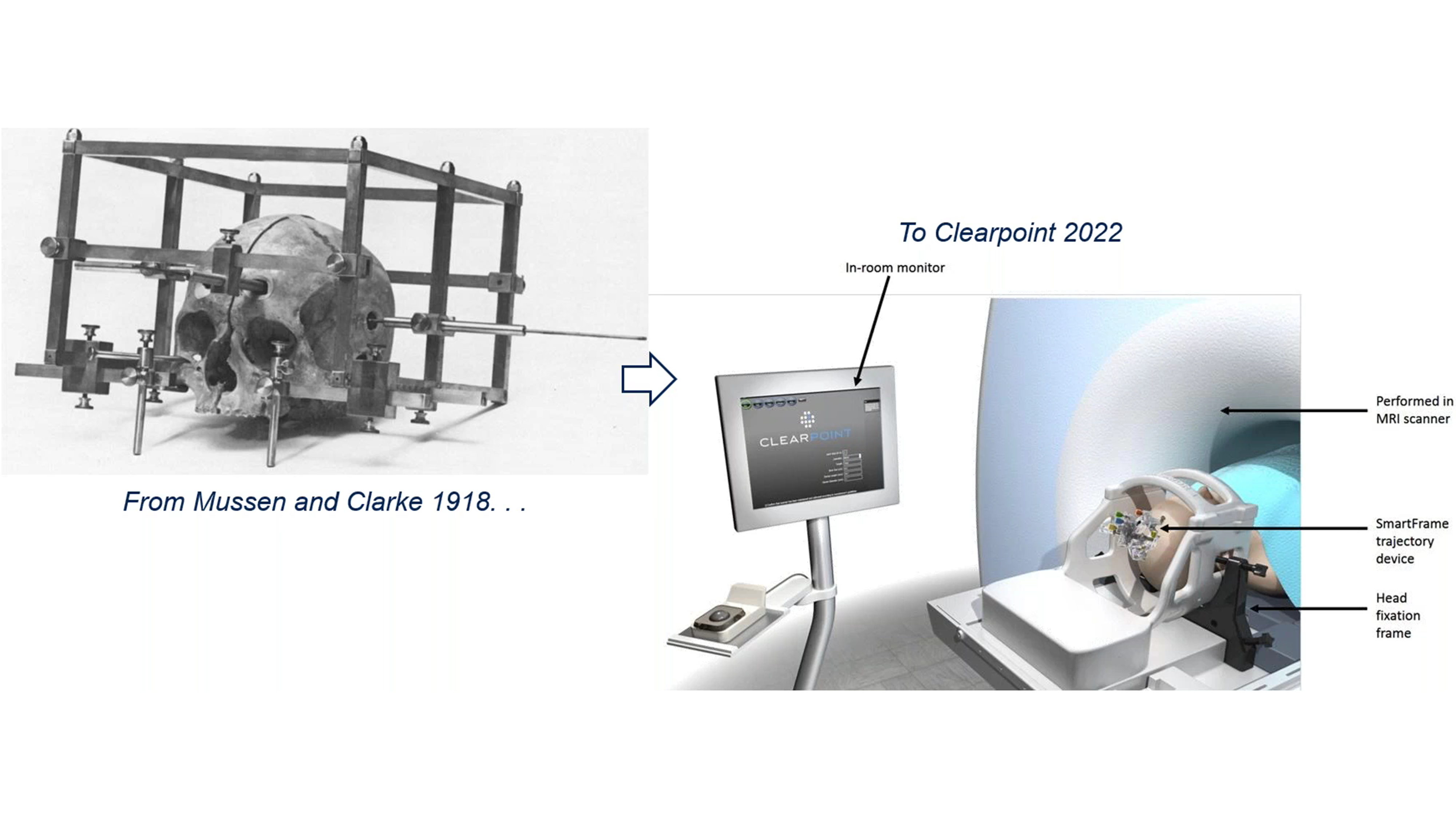
Figure 3. Progress in stereotactic techniques from the first human stereotactic frame to modern-day stereotactic guidance systems.
Stereotactic injection is a frequently used technique in neurosurgical research and this technology has the potential to drive how CNS therapeutics are delivered in the future.
Viral Vectors for Gene Delivery to the CNS
The use of viral vectors has become an important method for gene delivery to certain sites of the CNS. Several virus classes have been evaluated as vectors for gene delivery to the CNS, such as lentivirus, recombinant adeno-associated virus (AAV), retrovirus and more.
“Now AAV vectors are the delivery system of choice for gene delivery to the CNS as non-replicating systems,” explains Dr. Vornov. “Both intrathecal and stereotactic routes are being used to target CNS without systemic toxicity.”
Dr. Vornov explains how AAV uptake is widespread, but not homogeneous after intracisternal administration in animal models and there is currently no way to evaluate this in humans.
According to Dr. Vornov, stereotactic administration of AAV therapy has focused on areas of degeneration (for instance the striatum) and it is now known that there is trans-synaptic transmission of these vectors. Also, AAV strains vary in their transport to areas connected to the injection site and the thalamus is now being targeted to reach cortical areas more broadly. However, a drawback is that the dose and neuronal specificity of gene therapy is still poorly understood even in translational models.
Next Steps for Targeted CNS Delivery
Targeting the CNS directly has been successful in modern medicine. There are well-established techniques for both acute and chronic drug delivery to the CSF. Direct CNS administration is also FDA-approved for multiple small molecules and ASOs.
The next steps for targeted CNS delivery were reviewed by Dr. Vornov during the webinar and include the following:
- Chronic administration adapting current technology
- Optimization of dose and distribution to specific targets
- Reporter genes to understand dose and distribution
- The return of cell therapy-iPSCs (induced pluripotent stem cells) and genetically altered delivery cells
“The patients are waiting! Consider targeting your therapies that fail to be orally bioavailable and brain penetrant. I think we’re entering a new age here in CNS medicine that I hope will improve people’s lives,” concluded Dr. Vornov.
Modern-Day Applications of Direct CNS Administration
During the webinar, Dr. Rodrigues discussed specific considerations for intrathecal, ICV and intraparenchymal delivery of IPs. Out of these three techniques, intrathecal delivery is the simplest and the one with the most accumulated experience in clinical trials (Figure 4).
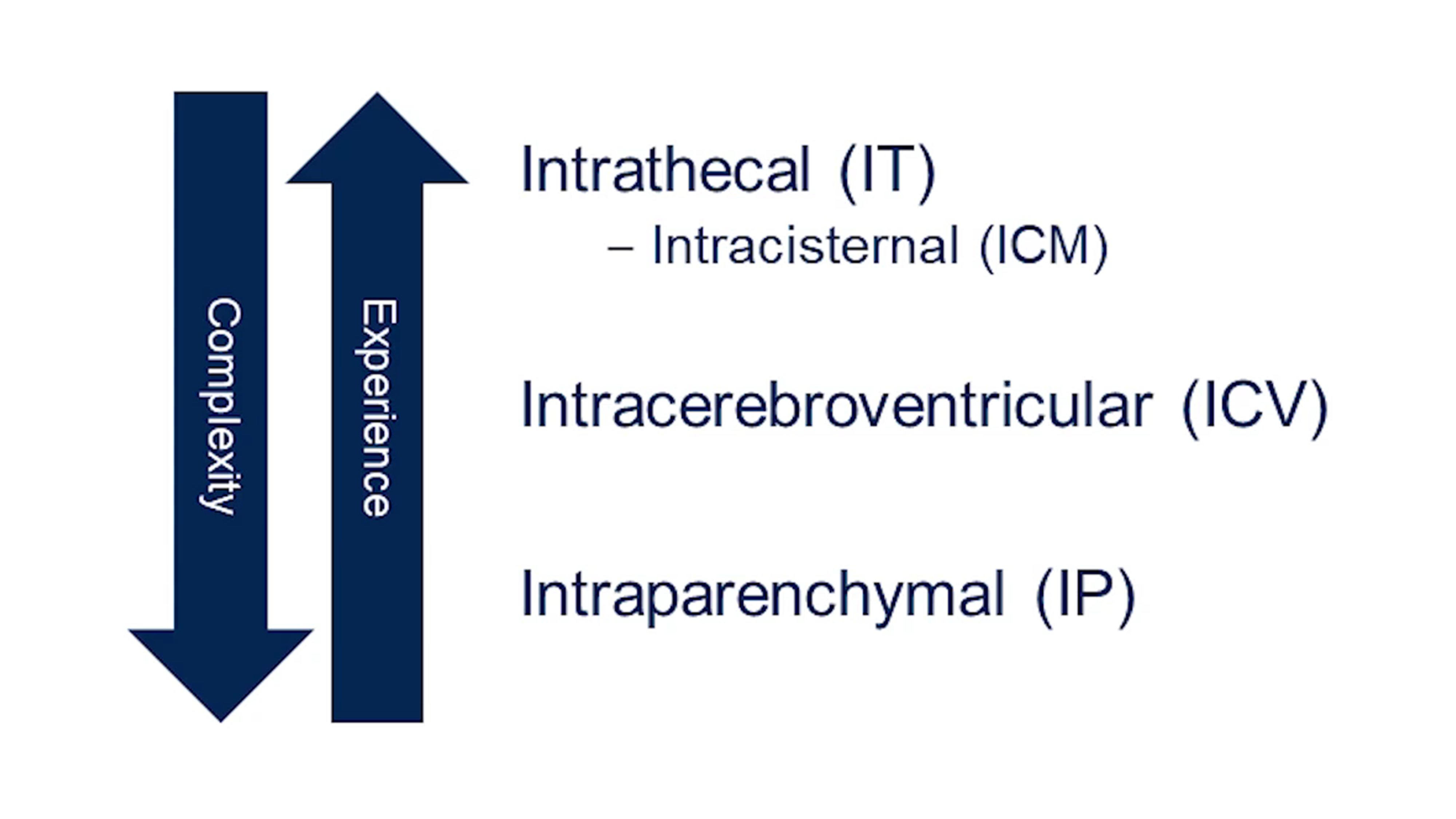
Figure 4. Comparing intrathecal, intracerebroventricular and intraparenchymal routes of administration of IPs.
ICV delivery instills the drug into the cerebral ventricles through an ICV device which consists of a port implanted under the scalp and an outlet catheter that leads to the ventricles within the brain. ICV delivery involves two surgical moments, one for the insertion and another one for the removal of the catheter and reservoir. The ICV method of drug administration usually involves experienced neurosurgeons, and it is likely the most labor-intensive technique compared to intrathecal and intraparenchymal delivery.
Intraparenchymal drug administration is the most complex out of the three techniques mentioned here, and intraparenchymal delivery involves neurosurgeons specialized in stereotactic surgery and more differentiated technical resources.
Considerations for Lumbar Spinal Intrathecal Delivery
During a lumbar puncture, a medical professional experienced with intrathecal delivery will insert a needle into the spinal canal between two vertebrae in the lower spine and inject the drug into the CSF (Figure 5).
According to Dr. Rodrigues, the research lumbar puncture procedure is very safe, is most often done under local anesthesia and is a very different experience from the procedure done in an emergency setting.
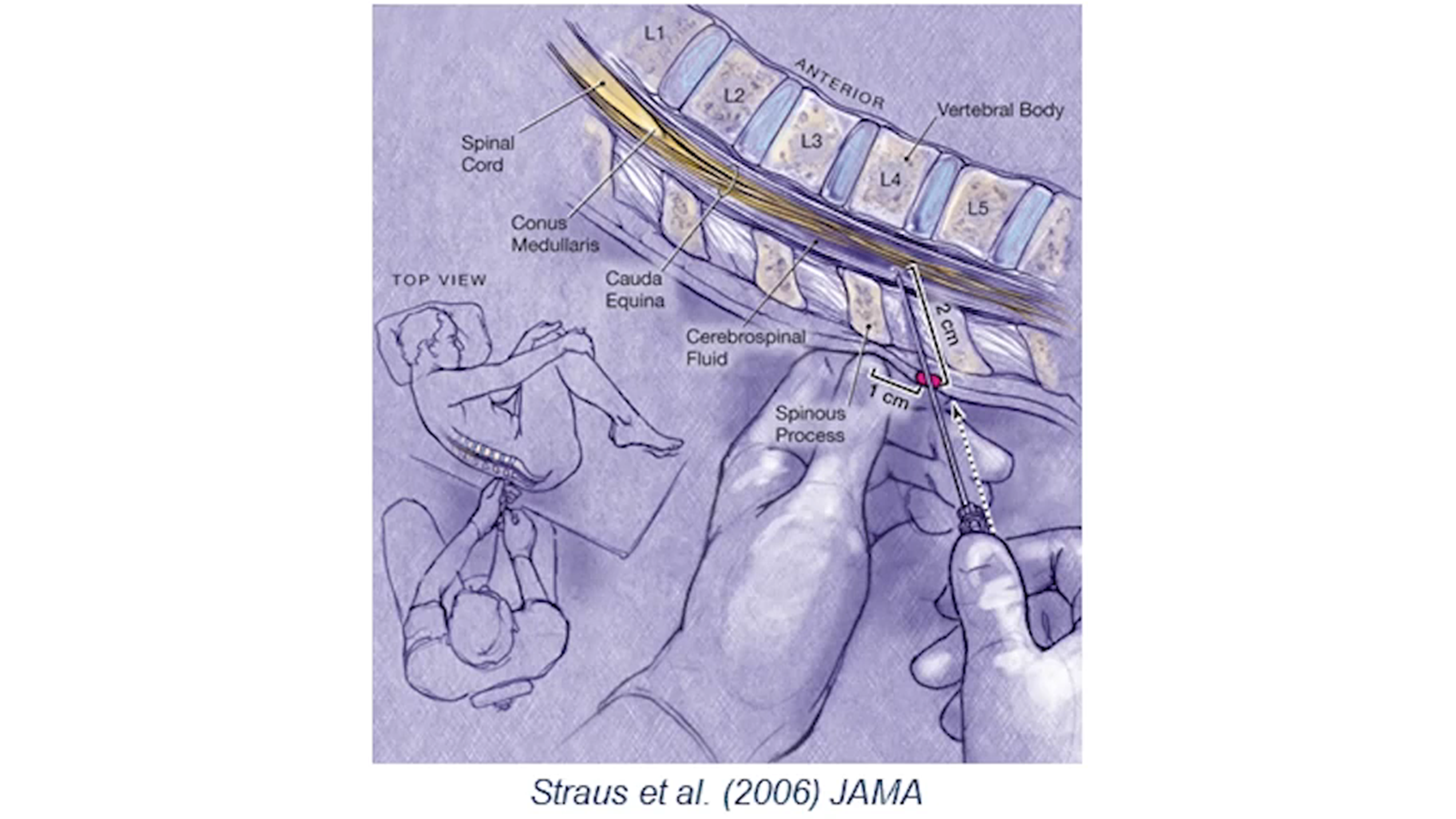
Figure 5. Lumbar spinal intrathecal delivery involves injecting the drug into the CSF.
Sites and researchers with lumbar puncture experience or training are desired for intrathecal drug delivery of IPs.
“Systems need to be in place to evaluate and manage complications, including access to an emergency department, imaging, spinal patches and in-patient facilities,” explains Dr. Rodrigues. “Ancillary services are also needed, such as a pharmacy to store, prepare and deliver the IP and labs such as a CSF lab to prepare samples.”
According to Dr. Rodrigues, the eligibility criteria for lumbar spinal intrathecal delivery are flexible, there are not many contraindications, and an experienced operator is successful in most circumstances. Participants also need to be informed about the procedure so they can understand what will happen and collaborate with the staff.
Before the procedure commences, there is a minimum set of requirements to guarantee safety, including a neurological exam and coagulation needs to be normal. Lumbar spinal intrathecal delivery can take around 10 to 20 minutes and the procedure can be done under imaging guidance to help inject the drug into the spinal canal.
Infection may be a complication from this procedure and patients may experience side effects such as back pain and headaches.
Post-procedural care may include a blood patch procedure and patients are typically recommended to get bed rest and hydration.
Considerations for Intracerebroventricular (ICV) Delivery
The ICV route of administration instills the drug into a lateral cerebral ventricle using an ICV port implanted under the scalp that consists of a reservoir and catheter (Figure 6).
ICV delivery needs a broad team, including a neurosurgeon who inserts the catheter and a team that administers the IP, which may be the same surgery team or a different team from the surgical team. It also involves more specialized facilities than those required for intrathecal delivery. For instance, catheter placement usually requires a surgical theater and neuronavigation devices.
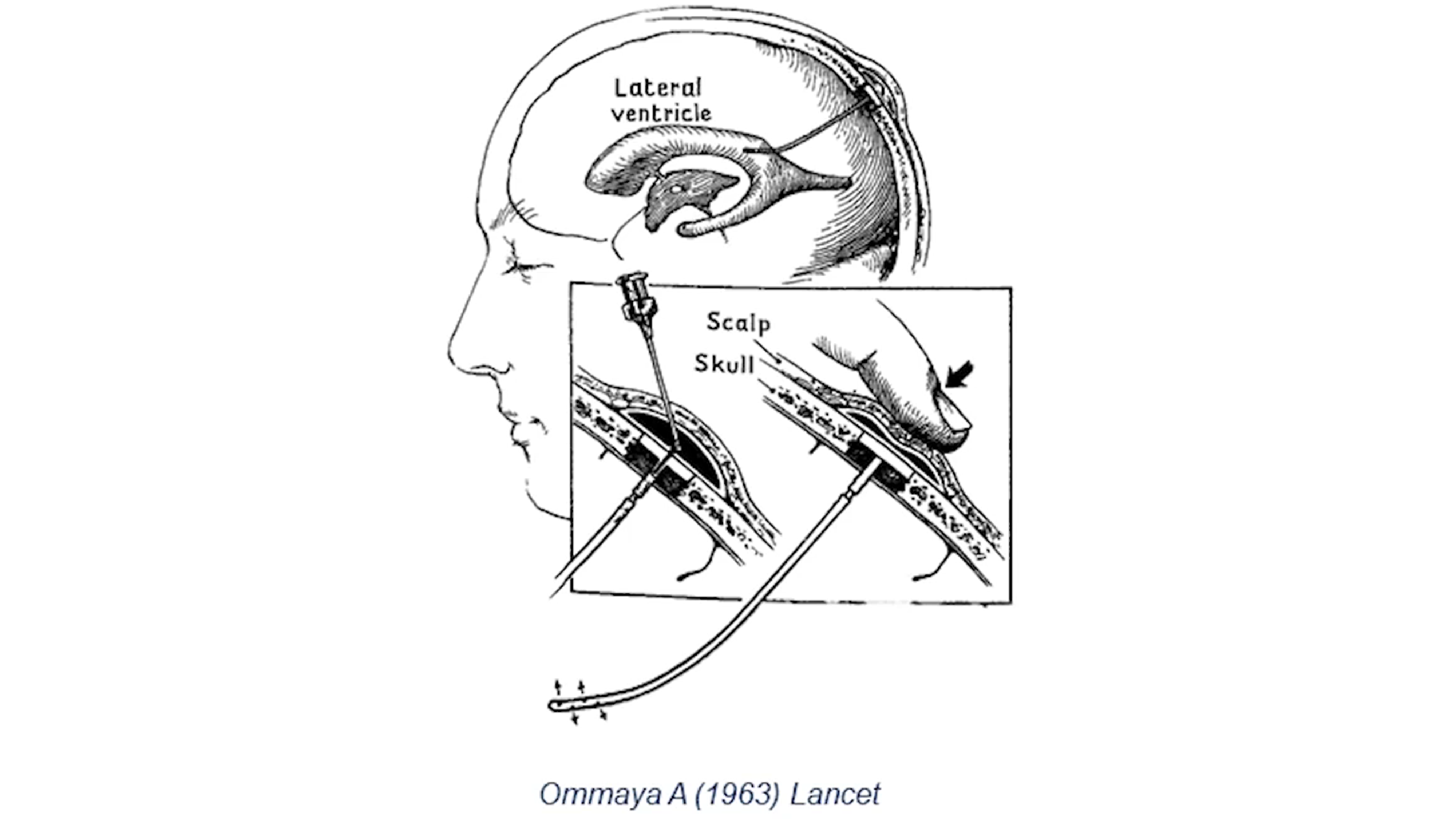
Figure 6. During ICV delivery, the drug is injected into a lateral cerebral ventricle and this procedure requires a neurosurgeon and a surgical team.
“In our experience, the most important risk mitigation aspect is the existence of an independent surgical committee that helps sites, principal investigators (PIs) and coordinators with surgical eligibility and planning aspects of the study,” says Dr. Rodrigues.
ICV delivery is always done under general anesthesia and this needs to be considered when selecting participants.
Dr. Rodrigues explains that brain imaging is essential to plan the ICV delivery procedure, so participants with contraindications are usually excluded. The surgical procedure is frequently preceded by an infection prophylaxis protocol since infection is an important potential complication. Also, for evident reasons, coagulation needs to be normal for this procedure to be conducted.
The ICV delivery procedure can take around 30 to 60 minutes.
“We always recommend using a reservoir for repeated injections, and that each injection is isovolumetric, meaning that the volume that is collected is equal to the volume that is injected,” explains Dr. Rodrigues. “After the surgery, the positioning of the catheter needs to be confirmed before the first injection, and usually the first dosing happens on day one post-op, which is frequently the same day patients are discharged from the hospital.”
As with the other two direct CNS delivery methods discussed, the teams must be aware of and know how to identify and treat potential complications, such as infection, bleeding, seizures and malfunctioning of the ICV port.
Dr. Rodrigues stressed that it is also important that the participant lives relatively close to a neurosurgical site, which doesn’t need to be the research site but a site with the capacity to identify and treat potential complications.
Considerations for Intraparenchymal Delivery
Intraparenchymal delivery is the most complicated of the three techniques discussed here and it involves directly injecting the IP into the brain matter (Figure 7).
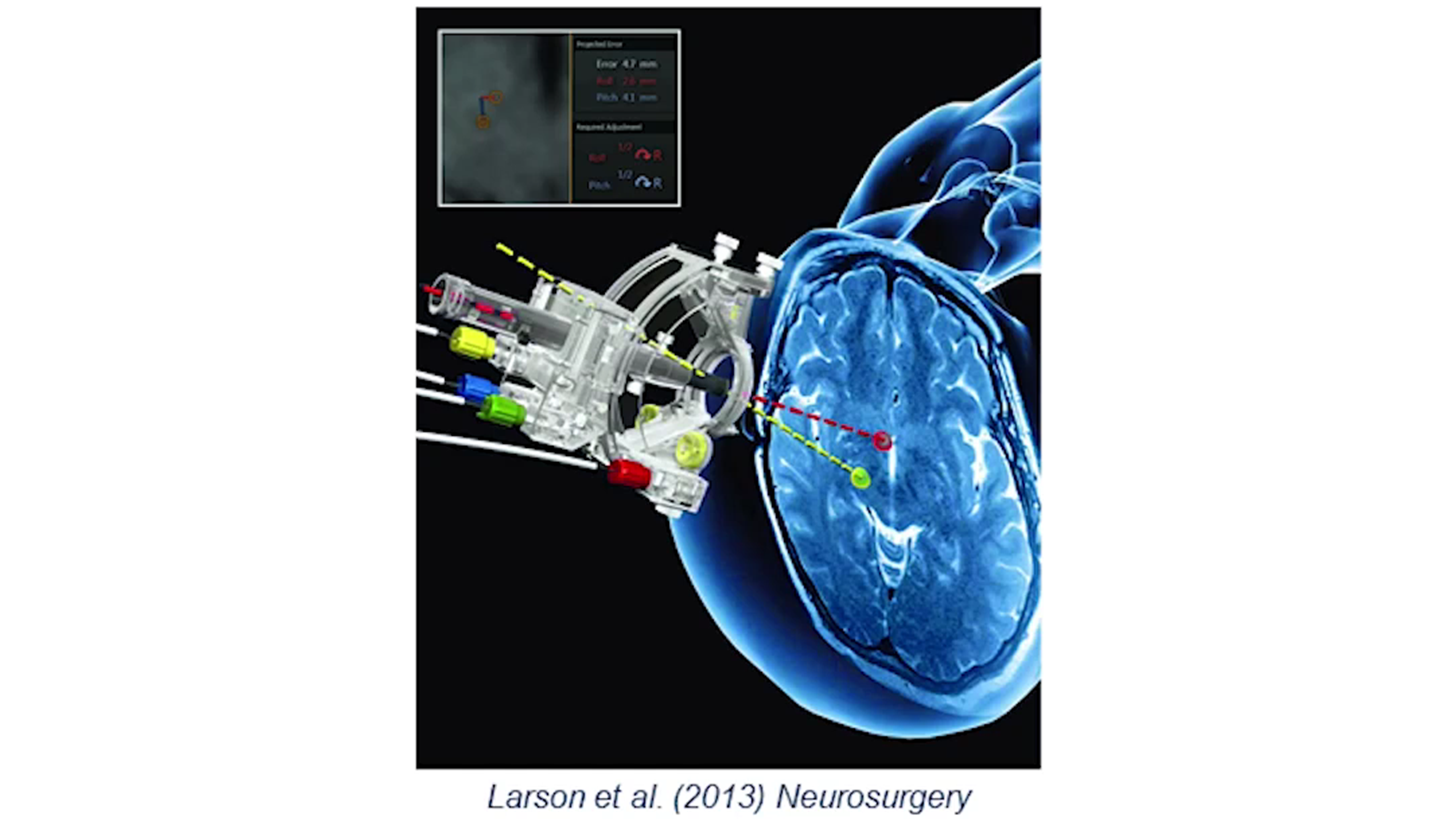
Figure 7. Intraparenchymal delivery involves infusing the drug directly into the brain tissue with a minimally invasive catheter. This procedure can take two to eight hours.
Intraparenchymal delivery is performed by a neurosurgical team who are experienced or willing to be trained in stereotactic brain surgery. The facilities required for intraparenchymal drug delivery include an operating theater with intratheater MRI scans and stereotactic equipment.
“Again, it is very important to involve neurosurgeons in development of the protocol and the study plans as is the existence of an independent surgical committee to help with eligibility and surgical planning,” says Dr. Rodrigues. “Because the procedure tends to be very long — up to eight hours — the anesthetic risk is a limiting factor, as is the actual volume of the targets being injected, and these are usually confirmed during the eligibility phase of the study.”
Pre-procedural care includes an infection control and prophylaxis protocol, and coagulation needs to be normal.
Intraparenchymal delivery has the unique characteristic of using convection-enhanced delivery, where the IP injection makes use of a concentration gradient and a pressure gradient to deliver the IP over a larger area of brain matter. Adding gadolinium to the IP provides a real-time understanding of where the drug is being distributed.
“A consideration is that for early-stage studies, the regulators usually are happier with a staged unilateral followed by a bilateral procedure to evaluate risks first,” explains Dr. Rodrigues. “Also, the control arm requirements and specifications depend very much on the geographic location and indication.”
Although there are potential procedural complications with this method of direct CNS administration, those are relatively rare in experienced and well-trained hands, according to Dr. Rodrigues.
After intraparenchymal delivery, the participants usually stay in the hospital for one to two days for observation because potential complications may occur, such as edema, infection, bleeding and seizures.
The key message from Dr. Rodrigues was that although these three techniques of direct CNS administration may add some complexity and risks, Medpace has plenty of experience implementing them and making their execution seamless, and Medpace is there to help.
Medpace’s Site Perspectives for Direct CNS Administration
During the webinar, Kelsey Carter discussed the investigative site perspectives that Medpace has for the execution of neuroscience trials with direct CNS drug delivery. In addition to the investigator and Medpace’s core site team, Medpace also has many departments to assist with neuroscience trials which use these methods of direct CNS administration (Figure 8).
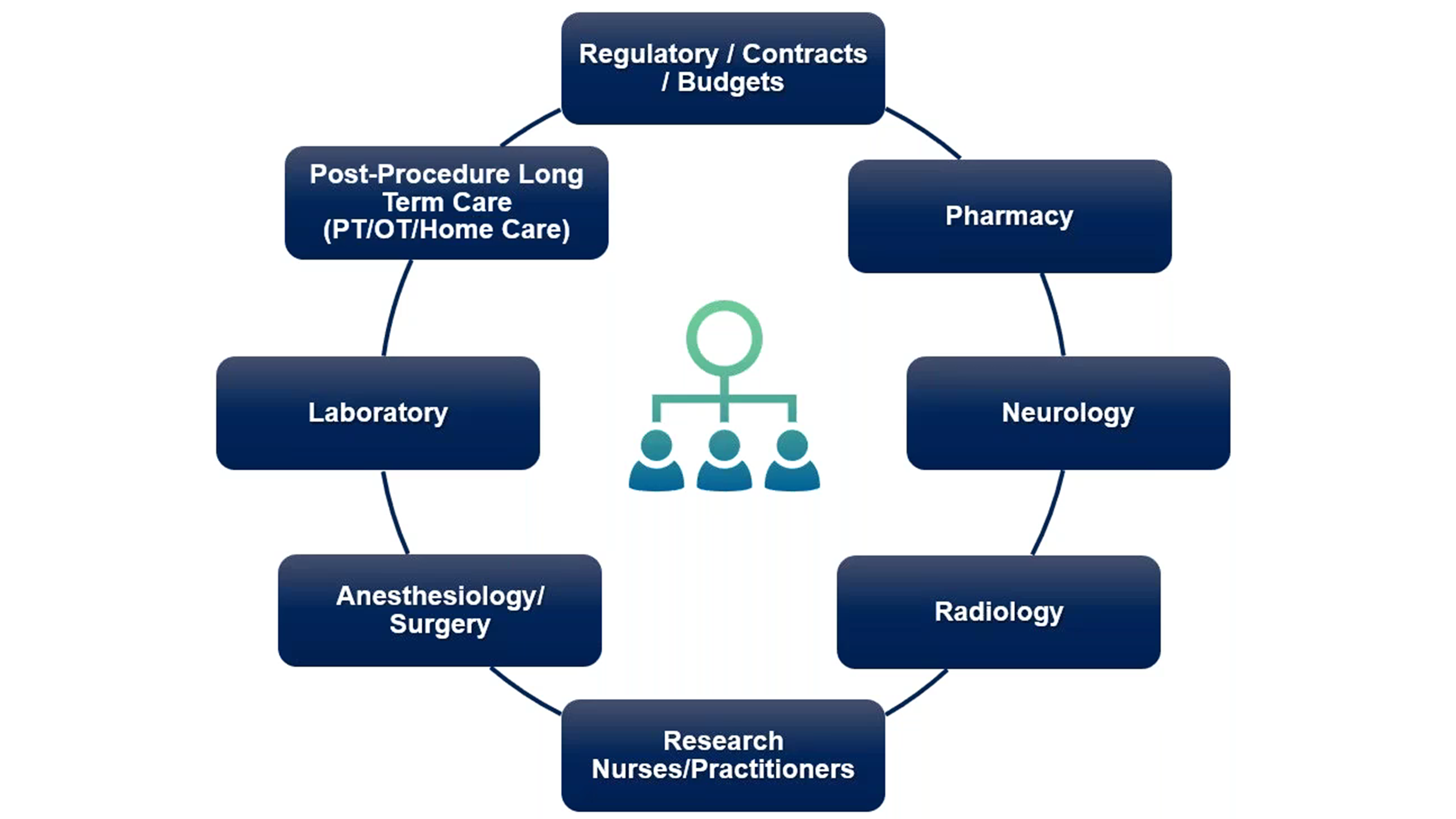
Figure 8. The site matrix for neuroscience trials with direct CNS administration. The main investigator core team is depicted in the middle.
The Regulatory/Contracts/Budgets team is a valuable part for setting up the study and understanding what other departments are needed based on the method of drug administration and the patient population.
The Pharmacy department prepares any medications needed for the operation as well as any pre-op and post-op medications. The Neurology and Radiology departments are vital for consultation and imaging, which is needed to establish baselines for a study.
Carter explained that Medpace certainly has a clinical team, but they may assign a dedicated research department with research nurses, practitioners or staff which is important if patients will need to be observed for a day or two after their procedure.
The Anesthesiology/Surgery team is needed for the direct CNS administration procedure. A Laboratory department is crucial if local or central CSF samples need to be collected and processed.
The Post-Procedure Long-Term Care team is important to ensure the participants feel supported prior, during and after any procedures. “We may need to consider physical therapy and occupational therapy teams, or even home care to support minimizing any post-procedure complications,” explains Carter.
In addition, Carter states that depending on the study setup, there could be blinded and unblinded site teams which could expand the site matrix of the contributing parties.
The study setup teams involved in neuroscience trials with direct CNS administration are shown in Figure 9.
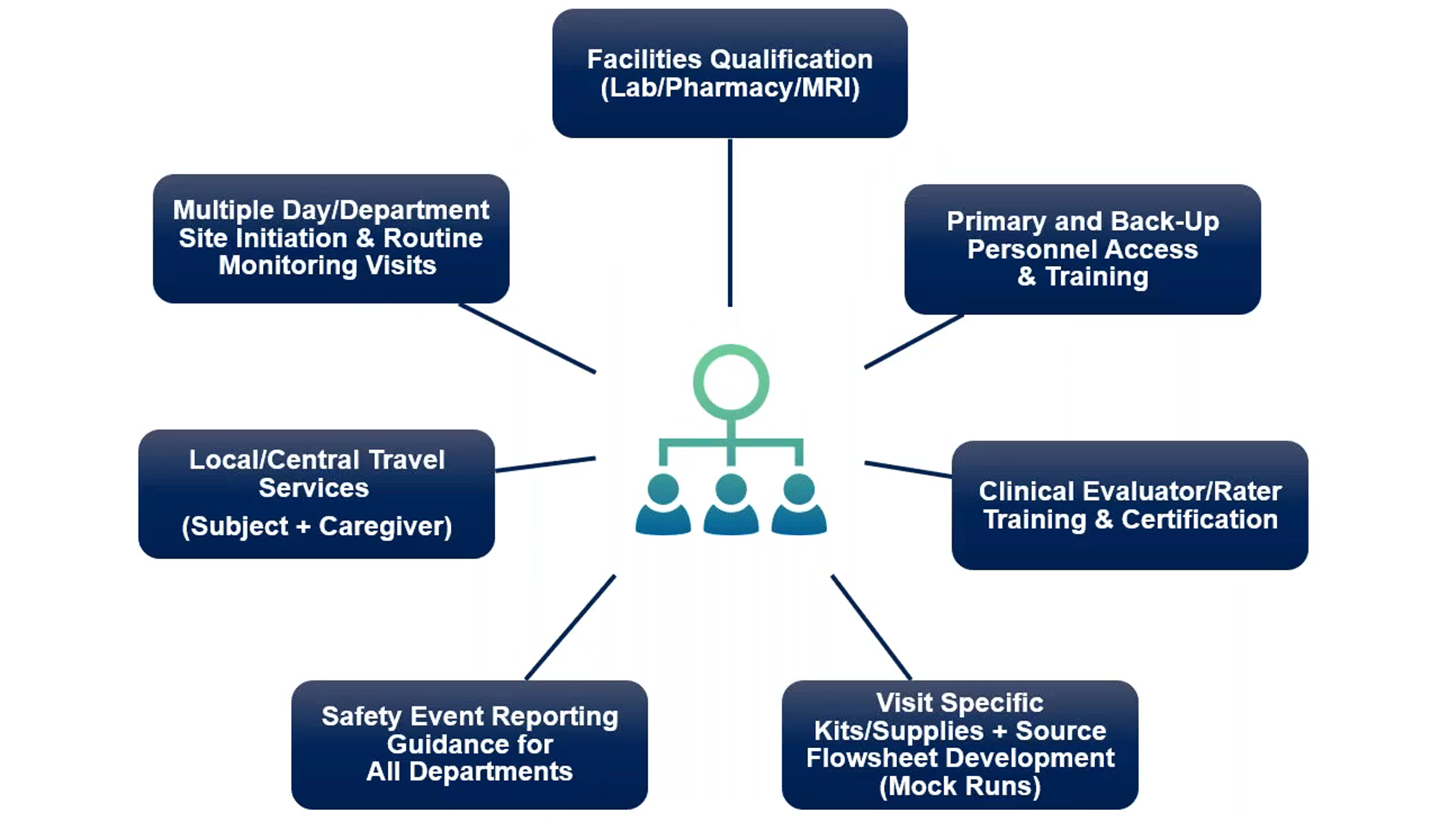
Figure 9. The study setup teams for neuroscience trials with direct CNS administration. The main investigator core team is depicted in the middle.
All facilities (such as lab, pharmacy, MRI, etc.) need to be qualified and have well-developed processes.
The primary staff and backup staff all need to be trained on the specific study procedures. Clinical evaluators and raters need to be trained and have the proper certifications. Also, participants like to see the same team members so ensuring consistency with the same team is important not only for the organization of the study but also for the participants.
“Visit-specific kits or supplies should be simplified as much as possible for the site teams and each visit needs to have a flowsheet source. In addition, a mock run may be implemented for process improvements and to minimize any rescheduling and burden on patients, caregivers and sites,” says Carter.
All departments need to be aware of their safety reporting requirements and it is useful for team members to have a department-specific checklist of what they need to document and report if something happens.
Local and central travel services ensure that the participant, their caregiver and any family part of the patient’s team have support getting to the clinical site, imaging site or surgical site. Such services help minimize patient burden and improve patient retention.
“Another consideration for study setup is that there could be multiple-day department site initiation,” says Carter. “In addition, routine monitoring visits are done to make sure that all team members are confident they can execute the study at the site level and that they are aware of any current updates.”
Medpace’s Operational Perspectives for Direct CNS Administration
During the webinar, Carter discussed the operational challenges of neuroscience trials with direct CNS administration. Carter spoke about how to mitigate challenges in study and site setup, the surgical procedure, complex IP management and administration requirements, gene mutation or speciality lab testing, CSF collection and vendor management.
Optimizing the Management of Studies and Sites with Intrathecal Drug Delivery
To alleviate challenges associated with study and site and set-up/management, lead site principal investigators with experience and well developed standard operating procedures (SOPs) should be included.
The number of surgical sites involved should be limited or combined clinical and surgical sites should be selected. It is beneficial to have good coordination among sites on surgical technique, results and lessons learned.
According to Carter, it is valuable to have dedicated neurosurgical, radiology and departmental staff for the surgery, MRI and other imaging techniques done at the clinical trial site. Overnight staff availability is also vital for patients who have overnight hospital stay.
Also, dedicated support is needed to coordinate surgical site set-up, training, cohort management and Data and Safety Monitoring Board (DSMB) meetings.
The key takeaway is ensuring that all team members are aware of the many moving parts needed for study and site management. “You can do this by coordination, planning, using checklists, flowcharts, well operationalized study manuals and rehearsals,” says Carter.
Mitigations for Surgical Challenges in Intrathecal and Stereotactic Delivery
According to Carter, a key surgical consideration not only for intrathecal but also for stereotactic delivery is ensuring there is a mock surgical run through the process and keeping in mind that there are planning committees which may require time and implementation of any observations from surgical planning committees.
Access to applicable subject data should be provided to the surgical team for their review in advance of pre-op visits.
Coordinating travel arrangements for the patients and their caregivers is beneficial for patient retention and it helps reduce patient burden. Travel arrangements should be made in line with site requirements for the pre-op and post-op procedure.
Scheduling post-surgical discussions between the surgical team and site staff who referred the subject for surgery is very beneficial, especially for complex stereotactic delivery procedures.
Alleviating Challenges with Complex IP Management and Administration Requirements for Intrathecal Delivery
Carter emphasises that one of the first things for sites to do for IP management is to lay out the communication pathway and transfer process between the pharmacy and clinical team for the management of the IP. Considerations include how to transfer the IP to the clinic and where to store it prior to administration.
Another thing to consider is that there needs to be protocol specification regarding coagulation/platelet testing prior to infusion.
Sties should be provided with IP administration and CSF collection manuals to outline the procedure and minimize infection risks.
For intrathecal administration of the IP, Carter says that additional guidance from neurologists and a dedicated lumbar puncture team may be needed. Neurologists may need more guidance about matters such as if atraumatic needles will need be used or if a large CSF volume must be collected.
It would also be valuable to provide fully equipped study supply kits to standardize the administration of the IP. In addition, an intrathecal injection expert may be identified and brought on to share best techniques or be consulted if sites have inquiries about intrathecal injections.
“Creating training video demonstrations of IP administration is also very helpful, especially if the IP administration is complex,” says Carter.
Staff also should be trained on managing post-lumbar puncture adverse events, such as headache, CSF leak, and others.
Mitigating Challenges with Trainings and Tracking, Speciality Lab Testing and CSF Collection
When it comes to mitigating challenges with scales setup as well as trainings and tracking, Carter says central vendors with training platforms are recommended. Rater visit scale packs should be prepared and distributed as it is important to make sure that raters and evaluators have the kit or packet they need when addressing patients as part of orchestrating the study execution and procedures.
For gene mutation or specialty lab testing, it is good to cooperate with the local or testing lab to confirm the mutation prior to screening and to provide the kits for genetic testing.
CSF collection may be a part of neuroscience trials with direct CNS administration, and so it is important to provide kits and specific CSF collection manuals to ensure continuity of collections while minimizing infection risks. Procedure-specific training for sample collections and handling will greatly help to ensure maintenance of CSF integrity and the interpretations of the results. There needs to be a guaranteed availability of any necessary specialty assays in addition to any required equipment and personnel for receipt of local results. Also, it is necessary to confirm CSF sample management, processing, storage and shipping at each site or lab location.
Diminishing Hurdles with Vendor Management for Studies with Intrathecal Delivery
With regards to vendor management, Carter explained that the operations team can support and make sure site teams have the best approach for some of these procedures by central specialty labs, as well as other study vendors.
Regarding central and specialty labs, clear expectations and guidelines should be provided for collection, processing, storage and shipment of all sample types. It is beneficial to provide a quick reference guide or flow chart including easy-to-use kits. A standard and holiday shipping schedule should be setup to ensure streamlined shipping of samples all year round. There needs to be proactive oversight on expected versus received samples, including final destination to specialty labs and final analysis. Carter also indicated that it is helpful to identify a single point of contact with regional support to oversee and drive lab deliverables to streamline communication and support accountability for each lab partner.
According to Carter, study vendors should be proactively managed with study-specific personnel for routine calls. There also needs to be a study communication plan with the vendors to detail responsibilities, oversight and risks. Regular transfer/reconciliation of vendor data is also required.
A key idea from Carter’s presentation is that feedback and process improvement from sites and study vendors can foster endpoint protection and positive participant outcomes.
The take-home message from the webinar is that clinical trials with direct CNS administration can be done and the technology is at a level that is mature enough for biotech companies to enter the field. Medpace is a CRO with experience in training investigators and teams to execute these complex trials.
To learn more about the considerations for neuroscience trials with direct CNS administration, register to watch the free on-demand webinar with Medpace’s clinical research experts.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.







Join or login to leave a comment
JOIN LOGIN