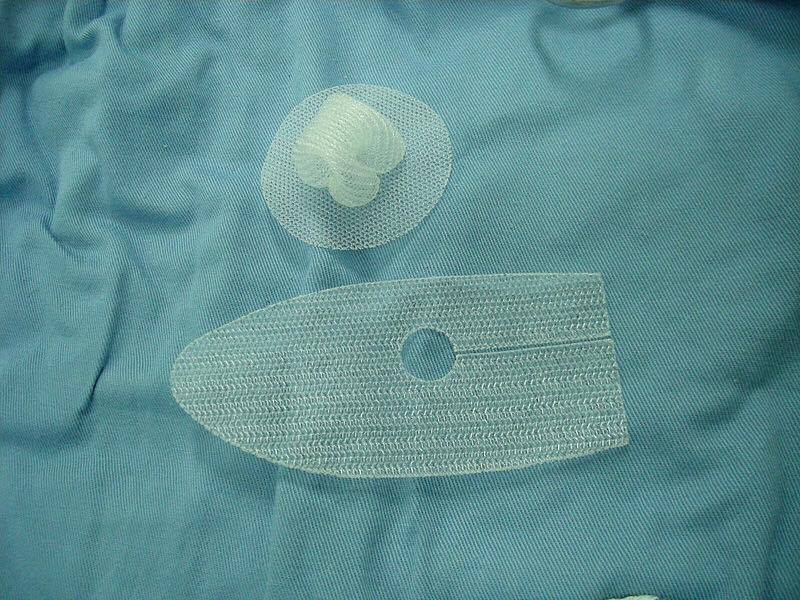
Synthetic surgical mesh is used in a variety of OR procedures, including hernia repair and fixing organ prolapse, however it’s fallen out of favor in recent years as some patients have reported serious complications including implant migration and chronic pain. The FDA has approved Surgical Innovation Associates’ DuraSorb Monofilament Mesh, a bioabsorbable device which can be used in reconstructive surgeries.
While conventional synthetic surgical mesh can act as an effective stabilizer for a variety of tissues, the risk of complications lies in its permanent presence in the body long after patients have undergone surgery. In some cases, the mesh may shrink or cause surrounding tissue to form adhesions and scar tissue that impact organ function and cause chronic pain. Unlike other medical devices, which sometimes have the ability to be taken out in the event of an adverse reaction, surgical mesh often becomes too embedded in patient tissue to allow for full removal.
It’s estimated that over one million patients in the US undergo a procedure involving surgical mesh implants each year. For patients requiring temporary tissue support during the healing process after these procedures, the DuraSorb Monofilament Mesh could provide an alternative to non-bioabsorbable devices by being completely dissolved into the surrounding tissue within one year of implantation.
“The idea of a mesh that is there when you need it and gone when you don’t is appealing, for much the same reason that absorbable sutures have become a key part of a surgeon’s armamentarium – tissue support from a foreign material is crucial during healing, but at some point thereafter may become a liability,” said Dr. John Kim, inventor of the device and Professor of Plastic Surgery at Northwestern University. “This technology was developed in direct response to unmet clinical needs in our field.”
In addition to providing a potential alternative to synthetic surgical mesh, bioabsorbable mesh could also replace certain biologic meshes which are composed of human or animal tissue harvested from cadavers. According to Surgical Innovation Associates, these biologic meshes can have a higher level of biocompatibility in the long-term, but they also come at a higher cost.
“Having known people who have gone through the pain of multiple mesh-related operations, I found it gratifying to collaborate closely with opinion-leading surgeons to make DuraSorb a reality,” says Alexei Mlodinow, CEO of Surgical Innovation Associates. “Their guidance went into every key decision during product development, and will now steer our clinical trial strategy as we replicate our robust preclinical data in a real-world setting.”
While choosing the DuraSorb Monofilament Mesh over conventional synthetic devices could mean a lower risk of post-operative complications for patients, it’s likely this bioabsorbable mesh won’t be appropriate for all procedures. Patients requiring longer-term tissue and organ support following reconstructive surgeries may still need the longevity of a synthetic mesh.
According to Surgical Innovation Associates, the biomaterial used to create their bioabsorbable mesh “has been safely used in other surgical applications for decades.” While it’s unclear exactly where in the US the DuraSorb Monofilament Mesh will first be made available, the company says it will start marketing the product early next year.










Join or login to leave a comment
JOIN LOGIN