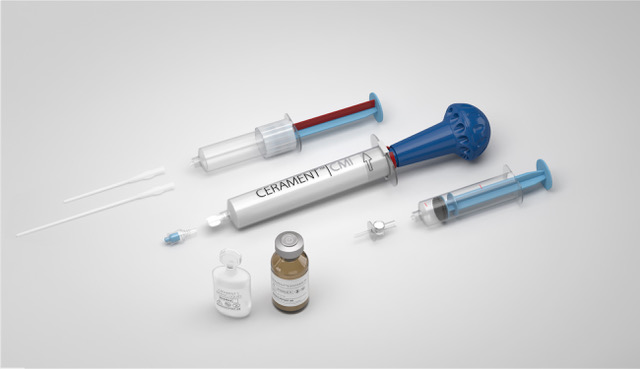
Ceramanet G, a bone void filler with the antibiotic gentamicin, fills bone voids and immediately begins bone healing with protection. Cerament G is a complete kit that allows for a single-stage surgery. Photo courtesy of BONESUPPORT.
Osteomyelitis (“osteo” meaning bone and “myelitis” meaning inflammation) is one of the most common acute and chronic bone infections that affects the bone and bone marrow at any age. In children and teens, the bones in the legs and arms are the most affected while in adults the spine and hips are the common targets of infection.
The disease can be grouped into three categories: a bone infection that spreads through the blood stream, an infection acquired after surgery or trauma and a foot infection in a diabetic individual or due to any other reason for a decreased blood supply to the bones.
Existing therapy techniques for osteomyelitis include surgery and antibiotics. In the case of long bone infections, a therapy of surgical removal of the infected soft tissue or bone and antibiotics is suggested.
Bone infections are caused by a wide spectrum of bacterial species such as Staphylococcus aureus, Proteus mirabilis, Salmonella enteritidis, Streptococcus pyogenes and Streptococcus pneumoniae. The patients would undergo a prolonged period of antibiotics therapy to completely eradicate the infection. Cerament G is a bone void filler treatment that can fill the gaps in the affected bones and simultaneously deliver the antibiotic gentamicin to reduce the risk of reinfection.
Cerament G — The Combination of Filler and Antibiotic
Cerament G is an injectable and moldable bone graft substitute. It comprises three components namely cerament powder (the sealant), saline solution and gentamicin powder (the antibiotic). Cerament powder is a blend of α-calcium sulfate hemihydrate (CaS) and hydroxyapatite (HA) in a ratio of 60 weight percent and 40 weight percent, respectively. Hydroxyapatite is a vital bone mineral, and it gives bone its rigidity. The hydroxyapatite was engineered to be a specific size and crystallinity which can help deliver the bone void filler in the form of an injection and allows for a slow resorption rate as well. Gentamicin is in a concentration of 17.5 mg/mL of cerament paste.
Hydroxyapatite has a beneficial trait of being osteoconductive which helps to strengthen the newly formed bone. Cerament G elutes gentamicin at levels above the minimum inhibitory concentration for at least 28 days, which lessens the chance of bone infection recurrence at the surgical site.

Cerament G was created for optimal management of bone gaps and voids. Photo courtesy of BONESUPPORT.
Bone Void Filler Cerament G: A Possible Healthcare Cost-cutter?
Currently, the healthcare industry is providing multi-stage treatment methods for patients affected by osteomyelitis and there can be a prolonged hospital stay. The US market approval of Cerament G can help transition the multi-stage treatment to a single-stage surgery treatment. The twin mode of action of bone void filling along with the supply of antibiotics to prevent bone infection recurrence can alleviate major healthcare costs.
The traditional treatment for osteomyelitis includes the placement of a non-absorbable antibiotic carrier by surgery with a subsequent healing period, followed by an autograft (harvest bone graft) procedure. With Cerament G, the healing starts right from the onset of the surgery where the filling and antibiotic delivery to the site is done in a single stage.
“The announcement from the FDA means that BONESUPPORT achieves the most important milestone in the company’s commercial history to date. The benefits of CERAMENT G for patients and clinics, have been validated in several very strong clinical trials, and have paved the way for our strong sales in Europe, where CERAMENT G accounts for a clear majority of our sales,” said Emil Billbäck, CEO of BONESUPPORT in the company’s press release.
XTALKS WEBINAR: How to Generate Patient Journey Insights by Leveraging Real-world Data from Electronic Health Records
Live and On-Demand: Monday, June 20, 2022, at 3pm EDT (12pm PDT)
Register for this free webinar to learn how to look beyond claims data to inform critical research. The session will feature a real client case study focused on the demographic and clinical characteristics over time of patients undergoing minimally invasive glaucoma surgery.
BONESUPPORT’s Bone Void Filler Cerament V
The orthobiologics innovator has developed yet another renowned injectable bio-ceramic bone graft substitute known as Cerament V. It has similar components as Cerament G but in the place of gentamicin, it has vancomycin. This product was designed to target vancomycin-sensitive microbes. Cerament V has already acquired its CE mark.
BONESUPPORT’s Ongoing Research
BONESUPPORT has emphasized that it has been involved in preclinical research to use a combination of Cerament with bisphosphonates (used to treat osteoporosis), bone morphogenic proteins (BMPs), bone marrow aspirate (BMA) and demineralized bone matrix (DBM).











Join or login to leave a comment
JOIN LOGIN