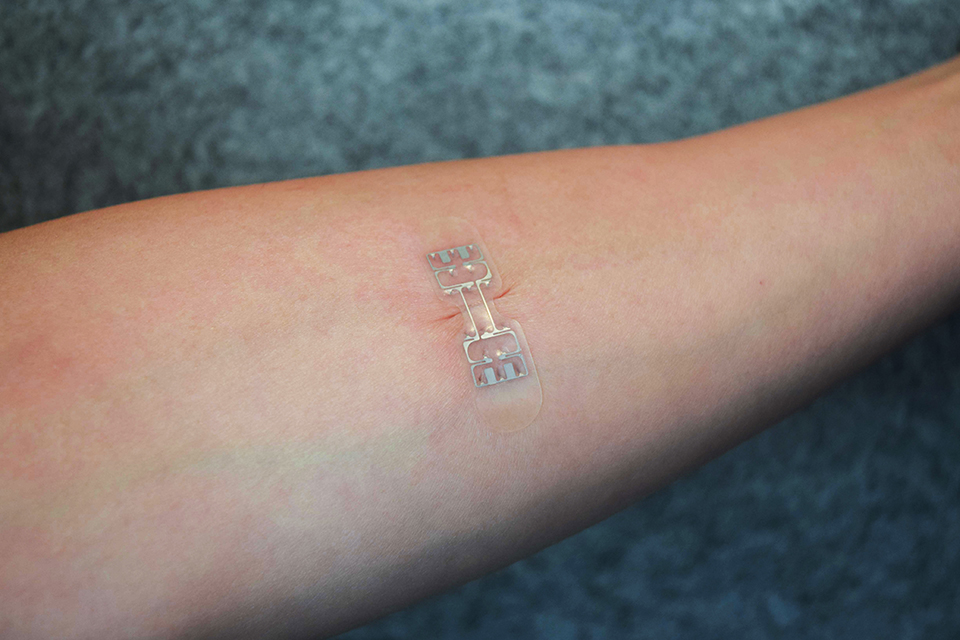
KitoTech Medical has designed a novel device capable of closing wounds and promoting healing without the need for surgical sutures or staples. The microMend skin closure device has the same adhesive properties of a bandage, but also includes two rows of tiny “microstaples” which help to bring the skin around the wound together.
Unlike traditional wound closure techniques, the microMend device is reportedly painless, and can be left on the patient for the duration of wound healing. The applications for the medical device include both accidental lacerations and surgical incisions.
“The market opportunity for microMend extends across a broad range of medical specialties, including surgery and emergency medicine. microMend will be initially promoted to dermatologists, who perform over 5 million surgical procedures, including excisions, skin biopsies, and Mohs surgeries,” said a press release issued by KitoTech Medical. The device was debuted at the Annual Meeting of the American Society for Dermatologic Surgery in early October.
In addition to offering many benefits to the patient – including faster wound healing and reduced scarring – the device could be a faster and easier way for hospitals to address wound closure. Currently, surgeons and other physicians must perform suturing after surgery as it requires certain skills and specialized techniques.
Since the microMend device requires little training for proper application, it could be applied by nurses and other healthcare providers, giving physicians more time for other tasks. Unlike non-dissolving sutures and staples, patients are able to remove the microMend device themselves, eliminating the need for return visits to their doctor’s office for removal alone.
A small clinical study conducted by KitoTech found that both doctors and patients preferred the microMend device to sutures. Samples of microMend were dispersed to dermatologists who attended the Annual Meeting of the American Society for Dermatologic Surgery, and KitoTech hopes the physicians will evaluate the device in their practices.











Join or login to leave a comment
JOIN LOGIN