Flow cytometric receptor occupancy assays are being increasingly used in preclinical and clinical studies.
Both the areas of drug development and clinical trials are increasingly using in vitro assays to help determine the efficacy of an investigational therapeutic.
Among these is the receptor occupancy assay, which is a powerful method used to understand how a therapeutic drug binds to (or occupies) its receptors on the surface of certain cells. Receptor occupancy assays can be coupled with flow cytometry to swiftly analyze individual cells in suspension. Generally, antibody-based therapeutics are one of the main types of drugs that are assessed using receptor occupancy assays as they bind to specific cell surface receptors through which they impact downstream intracellular signalling pathways.
All drug development processes evaluate a drug’s pharmacokinetics (PK) to gather data about its absorption, distribution, metabolism, and excretion in the body. The pharmacodynamics (PD) of the drug are also investigated to gain insight into the biochemical responses it elicits.
“Receptor occupancy assessments are sufficiently sensitive to monitor target binding and thus are very useful in drug development. Receptor occupancy assays have been developed and applied in both nonclinical and clinical studies to provide insight into PK/PD relationships so far,” said Andre Olsson, PhD, Senior Scientist, Flow Cytometry at the global contract research organization (CRO) Medpace. “Moreover, we can use receptor occupancy assays to provide valuable insights at all stages of drug development.”
In a recent webinar, Dr. Olsson described the benefits of flow cytometric receptor occupancy assays in clinical trials. In the same webinar, Dr. Priscillia Bresler, PhD, Scientist, Flow Cytometry at Medpace spoke about when receptor occupancy assays can be used and the requirements for implementing receptor occupancy assays in a clinical lab. The webinar also showed two examples of receptor occupancy assays conducted by Medpace for sponsors.
Watch the free, on-demand webinar to gain valuable insights from experts about the benefits of receptor occupancy assays in clinical trials.
What is Flow Cytometry?
Flow cytometry is an analytical technique that is used to detect the presence of cell surface markers as well as intracellular proteins using antibodies in individual cells suspended in a buffer solution, which pass through a laser beam one at a time (Figure 1). The antibodies are conjugated to fluorochromes for them to be detected. Fluorochromes are small molecules that are excited at a specific wavelength and emit light at other specific wavelengths. Over time, various fluorochromes were developed that can be detected at different wavelengths of light, allowing simultaneous detection of multiple fluorochromes on one given cell.
In addition to the light emitted by the fluorochromes, the forward and side scattered light from cells are detected by the flow cytometer. The forward scattered light, or simply the forward scatter (FSC), provides information about cell size, as the more light that is scattered, the larger the cell. The side scatter (SSC) is an indicator of cellular granularity; the higher the SSC, the more complex the cell is internally owing to organelles like the nucleus and granules.
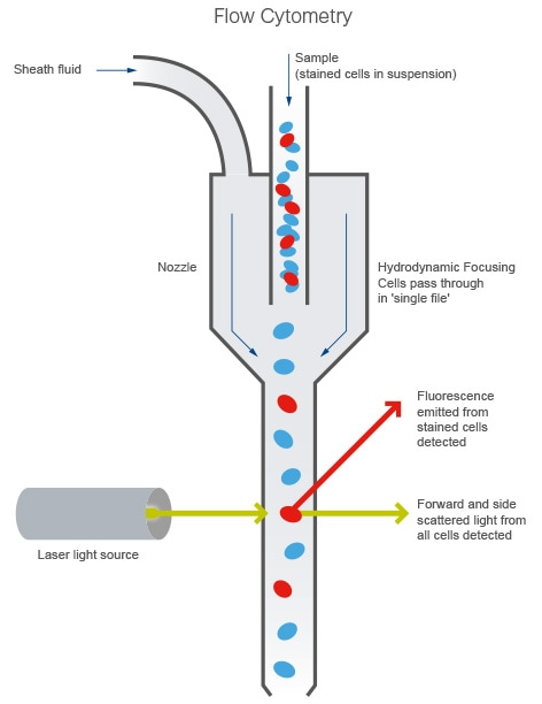
Figure 1. The flow cytometer. Figure from abcam.com.
A flow cytometer has four main components that include:
- Fluidics to align cells in a single cell suspension
- Optics, which include light sources to excite the fluorochromes and lenses and optical filters
- Detectors to detect forward and side scattered light as well as light emitted by fluorochromes
- Electronics to operate the instrument and capture signals from the detectors to produce data
How Can Flow Cytometry be Applied in a Clinical Lab?
Flow cytometry has been used in clinical settings for almost 40 years. The first clinical applications for flow cytometry started in the 1980s, including the assessment of CD4+ T cell numbers in patients with human immunodeficiency virus (HIV). HIV results in destruction of CD4+ T cells by binding to CD4 molecules on the surface of T cells to gain entry and replicate in them. Therefore, an accurate count of CD4+ T cells by flow cytometry helps determine the progression of the disease.
Even though individual cells may look similar under a microscope, they can have different surface receptors. For instance, all lymphocytes look the same when viewed under a microscope, but B lymphocytes (B cells) and T lymphocytes (T cells) have different cell surface receptor proteins. Because of this, antibodies can be used to identify the receptors that are specifically expressed on certain cells.
In multi-parameter flow cytometry, multiple antibodies can be used simultaneously to identify different cell populations. For example, four different cell populations can be identified using just two antibodies, each labelled with a different fluorochrome (Figure 2). The dot plot in Figure 2 has the signal intensity of each optical parameter represented on its own axis; four different cell populations appear in the dot plot.
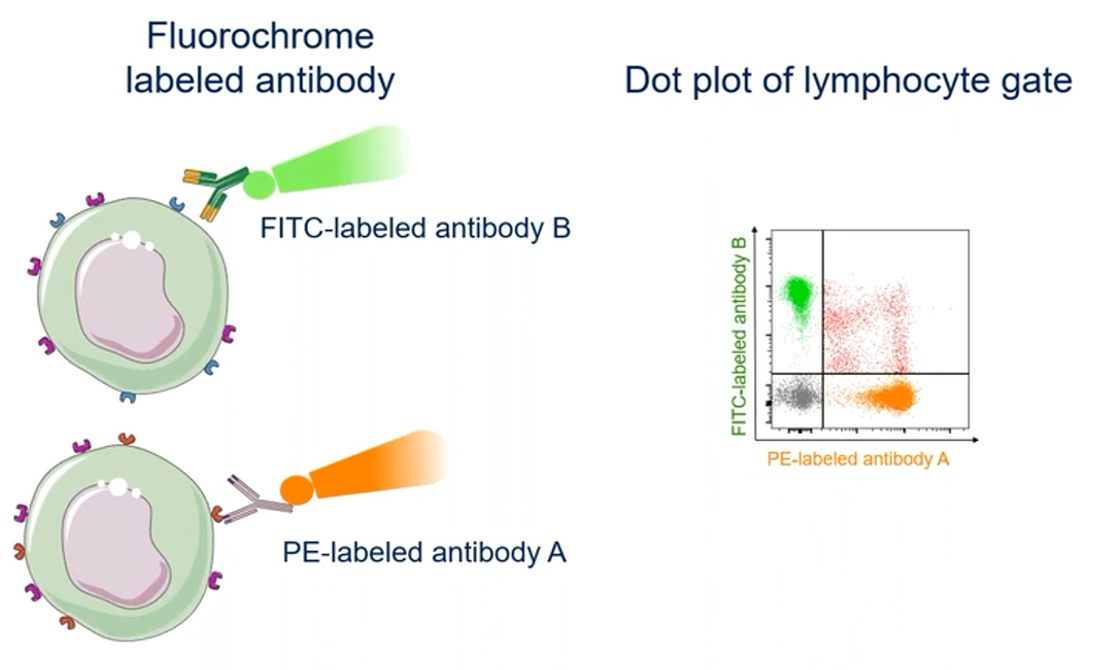
Figure 2. Combining fluorochromes and antibodies to identify different cell types.
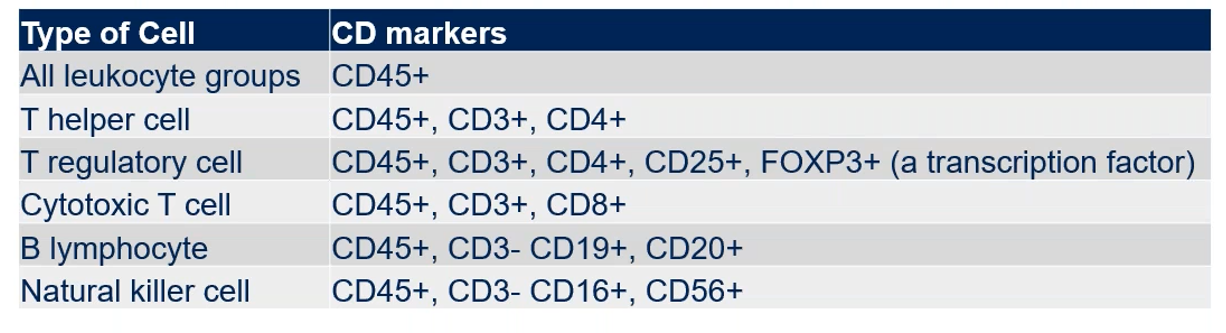
Cells express different protein markers on their surface, which can be used to help in their identification. For example, CD (cluster of differentiation) markers are associated with different cell lineages, and a combination of several CD markers is typically used to delineate cell subtypes more accurately within a pool of cells. A panel of multiple CD marker antibodies is usually used to profile and ID specific cell types among a heterogenous sample of cells using flow cytometry. Examples of immune cells and the CD markers they express are shown in Table 1.
Table 1. CD marker surface expression on cells of the immune system.
“In the clinical lab, the main power of the cytometer is its precision and accuracy,” said Dr. Olsson. “We can look at heterogenous samples, such as blood, bone marrow, or any fluid that has cells in it, and we can specifically identify cells within that heterogeneous sample. With that, we can do a relative quantification and we can do an absolute cell count by cell enumeration.” Dr. Olsson explained that in one in vitro diagnostic assay that they run, CD4+ and CD8+ T cells are profiled, which is useful for tracking the progression of HIV.
Dr. Olsson described that hematologic disease processes can be profiled by labeling for various CD markers. “For instance, we can track B cell malignancies over time by using B cell markers. We can use stem and progenitor cell markers and track myeloid malignancies over the course of the study.”
In addition, Dr. Olsson explained that cell sorting by flow cytometry can be used in the clinical laboratory to isolate a specific cell type of interest for analysis by PCR or single cell RNA sequencing.
What is a Receptor Occupancy Assay?
A receptor occupancy assay is used to assess the degree of binding of a biotherapeutic agent to its extracellular target, which is usually a cell surface protein. Usually, these drugs are monoclonal antibody therapeutics that target specific cell receptors to modulate intracellular signalling pathways, primarily by blocking the binding of endogenous ligands to the receptor. Flow cytometry is the primary analytical technique used to measure receptor occupancy of therapeutic compounds.
“By performing a receptor occupancy assay, we will be able to know what percentage of receptors are occupied by the drug, if the drug is efficient and specific or what would be the mechanism of action of the drug. To reply to these questions, we can use the flow cytometry technique and at Medpace Laboratories, we have experience performing flow cytometry assays, like receptor occupancy,” said Dr. Bresler.
There are three types of receptor occupancy measurements — free receptor, bound receptor, and total receptor — that can be performed to evaluate the percentage of receptor occupancy in clinical trials.
The free receptor occupancy measurement shows the receptors that are not bound by the antibody drug being evaluated. Determining the percentage of free receptor is valuable to assess cell specificity and the therapeutic doses of an antibody. There are two methods to measure the percentage of free receptors, and both require ex vivo saturating conditions with the unconjugated drug: one method uses a competitive antibody that is fluorescently labelled (Figure 3A) and the other uses a fluorescently labelled drug (Figure 3B).
For free receptor measurement with a competitive antibody (Figure 3A), it must be fluorescently labelled and must bind to the same epitope as the drug does. “For the competitive antibodies used, it’s really important to make sure that the specificity of this antibody is equal or lower than the therapeutic drug. Otherwise, the competitive antibody will displace the conjugated drug and then you will underestimate the calculation of the free receptor occupancy,” explained Dr. Bresler.
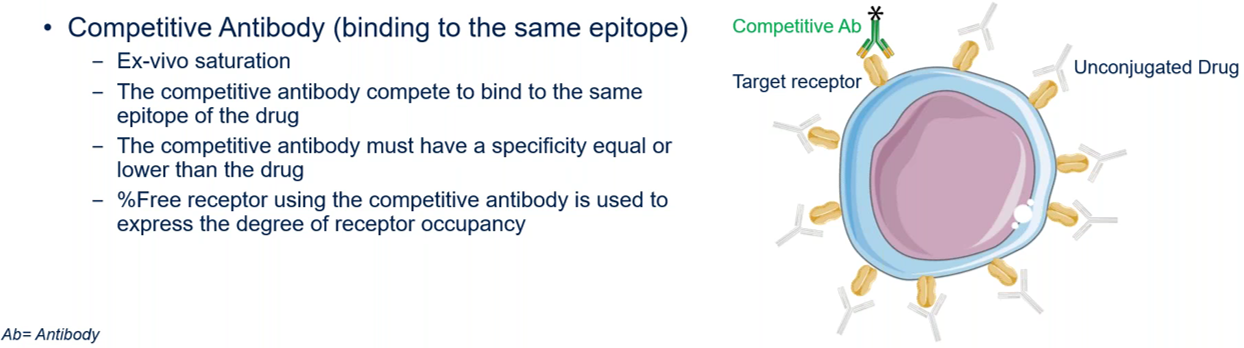
Figure 3A. Measurement of free receptors using a competitive antibody that is fluorescently labelled.
The percentage of free receptors can also be calculated using a fluorescently labelled drug (Figure 3B). The signal it generates indicates the degree of receptor occupancy and is used in the calculation of the percentage of free receptors. The advantage of this method is that there is no competition; however, it may be challenging to conjugate the fluorescent label to the drug while keeping acceptable specificity.
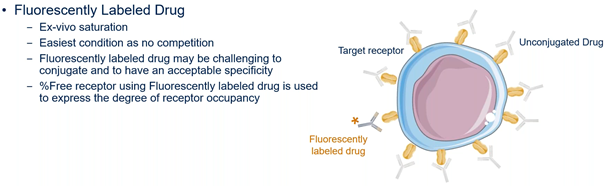
Figure 3B. Measurement of free receptors using a fluorescently labelled drug.
Another type of measurement is the percentage of bound receptor, which involves direct measurement of therapeutic antibodies bound to the target receptor (Figure 4). This assay uses secondary anti-drug antibodies that have a fluorescent label, which will bind to the drug that is bound to the receptor of interest. This approach is used when fluorescently labelled antibodies against the receptor are not available or if they don’t yield a good signal.
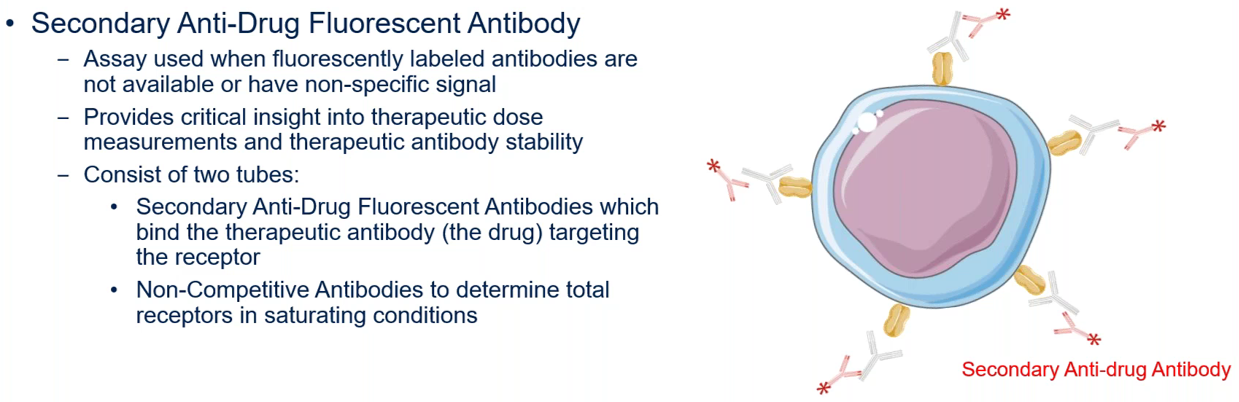
Figure 4. Measurement of bound receptors using a secondary anti-drug fluorescent antibody.
A third type of receptor occupancy measurement called total receptor measurement, which measures all receptors independent of the presence or absence of the drug (Figure 5). This measurement provides insight into the level of receptor internalization that results from binding of the drug. This assay uses fluorescent secondary anti-drug antibodies to measure the binding of the drug to its receptor site. In addition, this assay uses fluorescently labelled non-competitive antibodies to bind to the totality of the receptors, regardless of whether the drug is present or not. These non-competitive antibodies work by binding to a different site on the same receptor.

Figure 5. Measurement of total receptors using non-competitive fluorescent antibodies and secondary anti-drug fluorescent antibodies.
The selection of which receptor occupancy assay to use depends on several factors, such as the availability of reagents and the mechanism of action of the drug. In addition, more than one type of assay may be used, depending on the question being investigated. When calculating the degree of receptor occupancy, the researcher must use the appropriate receptor occupancy equation.
When Can Receptor Occupancy be Used?
Receptor occupancy data from laboratory animals or human cells along with PK/PD data can support the preclinical and clinical phases of the drug development process. Receptor occupancy assays can be used in preclinical toxicology studies to confirm target engagement. These assays can also be used in PD measurements together with PK data to optimize clinical trial design and guide dose selection.
In addition, receptor occupancy assays can be used to assess the impact of neutralizing antidrug antibodies (ADA) on receptor engagement. Neutralizing ADAs bind to the drug and prevent the drug from binding to its target. This is done to evaluate the potential of the ADA to inhibit the pharmacological function of the drug.
What is Needed to Implement a Receptor Occupancy Assay in a Clinical Lab?
A flow cytometric receptor occupancy assay needs to be carefully designed, validated, and optimized. Dr. Bresler provided a detailed review of some important assay considerations in the webinar, which researchers should look into when incorporating receptor occupancy assays in clinical labs. Topics that were discussed by Dr. Bresler include:
The design input stage. This is the first step of a receptor occupancy assay. It involves understanding the biology of the cell populations of interest and asking questions such as:
- What is the cell phenotype?
- How does the expression of the cells compare in a healthy versus disease state?
- What is the in vivo localization of the cells?
The design process stage: This stage involves considerations for successful measurement of receptor occupancy by flow cytometry and includes:
- Pre-analytical considerations for the specimen type, which is most commonly peripheral whole blood
- The selection of cell markers, fluorochromes and antibodies
- Careful sample preparation and sample stability assessments
- Finding the optimal saturating conditions as all receptor occupancy antibodies must be used at a saturating concentration
- Optimizing the flow cytometry instrumentation and using a gating strategy to enhance the resolution of the cell population of interest
The success of a receptor occupancy assay depends on the work of knowledgeable researchers. The flow cytometry team at Medpace is led by PhD-level scientists with over a decade of experience in the design, data analysis and interpretation of multicolor flow cytometry assays.
The Benefits of Receptor Occupancy in Clinical Trials
In the webinar, Dr. Olsson explained that receptor occupancy assays are valuable for all stages of the drug development process because these assays:
- Provide information about the mechanism of action and binding characteristics of the drug to its target receptors
- Are important during Phase I clinical trials to inform about therapeutic doses
- May be required together with PD and PK data to guide dosing protocols for the identification of the biological effect
- Are informative for Phase II clinical trials to predict the levels of receptor occupancy and determine if the receptor is modulated on cells targeted by the drug
- Have been used as a PD biomarker for several therapeutic antibodies, including anti-PD-1, AMG479, ATR-107 and Etrolizumab
Therefore, receptor occupancy assays have been used in both nonclinical and clinical studies to support the understanding of PK/PD relationships.
Receptor Occupancy Measurements at Medpace
“At Medpace, we have seen an upswing in the receptor occupancy assays that we have been awarded in the last two years,” said Dr. Olsson.
During the webinar, Dr. Olsson provided two examples of receptor occupancy data that Medpace has measured for sponsors.
The first example involved data which shows the efficiency of a drug, with 100 percent receptor occupancy reached at the start of the study and maintained at high levels throughout the course of the study.
The second example was an exposure-response analysis using a sigmoid Emax (Effect Maximal) model, which showed that receptor occupancy has a dose-dependent relationship with drug concentration; this modelling was used to establish the EC50 (Effect Concentration), which is the concentration of the drug required to obtain a 50 percent effect.
Receptor occupancy assays are being increasingly used in PK/PD modeling and recommended to ensure the effectiveness and specificity of a therapeutic.
Frequently Asked Questions About Receptor Occupancy Assays
The recent webinar by Medpace had a Q&A session where Dr. Bresler and Dr. Olsson addressed questions attendees had about receptor occupancy assays. Below are some interesting questions from that session along with the answers provided by the experts at Medpace.
How long does it take to develop receptor occupancy assays, from conception to validation?
Dr. Bresler: The scientists at Medpace develop and optimize receptor occupancy assays from scratch or can cross validate a protocol previously established by the sponsor. This process takes 14 to 16 weeks. In addition, some time is needed to conduct feasibility experiments to verify that there is a decent number of cells targeted by the drug for good sensitivity.
How is stability determined during Receptor Occupancy validation?
Dr. Bresler: The determination of sample stability is critical for RO assays and must be part of the assay validation. The stability is simply tested by calculating the %bias of RO (and other reportables if necessary) between a freshly drawn sample (baseline) and different timepoints from the same specimen. The stability can be determined before processing and after processing. The comparison between 4°C and room temperature can be performed as well to make sure to store the samples in good condition. Usually, the acceptance criteria is 20% bias compared to baseline.
How is the detection method decided in receptor occupancy?
Dr. Olsson: You can look at your free receptor or your bound receptor, and you can perform accuracy and precision [tests] on that and see which one performs the best. Then go with the free receptor if that has greater accuracy or go with the bound receptor if that has greater accuracy. It mostly comes down to precision and accuracy.
Can the binding receptor occupancy data be used as a good marker for efficacy — can it be used to personalize the dose for a patient, to have a more efficacious and safer dose for a patient?
Dr. Olsson: On its own, the receptor occupancy assay can not be used for efficacy; however, when coupled with other data, one can customize the dose response of the subject. This is because as soon as a receptor occupancy assay is done and the data is analyzed, there is an answer on the level of the receptor occupancy assay.
If the target cells are on tissues but one wants to use peripheral blood mononuclear cells (PBMCs) as surrogate, what needs to be done?
Dr. Olsson: If you have your target cells on any type of cell that is within PBMC, we do the whole validation on the PBMCs because, as Dr. Bresler indicated, where you isolate PBMCs, you process the cells and that might change how the receptors behave. If the receptor occupancy assay is established in whole blood, for instance, but because of reasons in the study it can only be performed on PBMCs, we have to perform a validation and establish that our criteria is upheld on PBMCs just as they were on whole blood — we can’t just assume that it will work on PBMCs because it worked on whole blood.
How can receptor occupancy assays be used to clinically interpret values in clinical studies?
Dr. Olsson: The receptor occupancy assays must be correlated to safety data or the PK or PD data that are associated with the study. On its own, the receptor occupancy is a number which can be used to determine whether the drug is at saturated or non-saturated levels; however, that must be correlated with safety data or other type of data in the study.
To learn more about the development and benefits of flow cytometric receptor occupancy assays for clinical trials, register to watch the free on-demand webinar by Medpace.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.
REFERENCES
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454.
Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, Murphy B, Roth B, McCaffery I, Gorski KS, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol 2009;27:5800–5807.
Hua F, Comer GM, Stockert L, Jin B, Nowak J, Pleasic-Williams S, Wunderlich D, Cheng J, Beebe JS. Anti IL21 receptor monoclonal antibody (ATR-107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol 2014;54:14–22.
Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, Schreiber S, Mansfield JC, Williams M, Tang M, et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013;62:1122–1130.
Thway TM, Magana I, Bautista A, Jawa V, Gu W, Ma M. Impact of Anti-Drug Antibodies in Preclinical Pharmacokinetic Assessment. AAPS J 2013;15:856–863.
Audia A, Bannish G, Bunting R, Riveley C. Flow cytometry and receptor occupancy in immune-oncology. Expert Opin Biol Ther 2021;22:87-94.
Green CL, Stewart JJ, Högerkorp C, Lackey A, Jones N, Liang M, Xu Y, Ferbas J, Moulard M, Czechowska K, et al. Recommendations for the development and validation of flow cytometry-based receptor occupancy assays. Cytometry B Clin Cytom 2016;90B:141-149.
Wang R, Gao C, Raymond M, Dito G, Kabbabe D, Shao X, Hilt E, Sun Y, Pak I, Gutierrez M, et al. An Integrative Approach to Inform Optimal Administration of OX40 Agonist Antibodies in Patients with Advanced Solid Tumors. Clin Cancer Res 2019;25:6709-6720.
Hilt E, Sun YS, McCloskey TW, Eck S, McIntosh T, Grugan KD, Lanham DF, Standifer N, Green C, Litwin V, et al. 2021. Best practices for optimization and validation of flow cytometry-based receptor occupancy assays. Cytometry B Clin Cytom 2021;100:63-71.
Sternebring O, Alifrangis L, Christensen TF, Ji H, Hegelund AC, Högerkorp C. A Weighted Method for Estimation of Receptor Occupancy for Pharmacodynamic Measurements in Drug Development. Cytometry B Clin Cytom 2016;90B: 220-229.
Recommendations for Flow Cytometry-based Receptor Occupancy (RO) Assays. (Posted on June 10, 2021). FlowMetric a KCAS company. https://www.flowmetric.com/cytometry-blog/receptor-occupancy-assay-recommendations






Join or login to leave a comment
JOIN LOGIN