Cardiovascular clinical trials need to have a diversity plan because there can be a difference in disease burden when comparing people of different race and ethnicity.
The US Food and Drug Administration (FDA) is emphasizing the need for diversity in clinical trials. This started back in 1993 when the FDA established guidelines to increase diversity by gender and race/ethnicity of participants in clinical trials that were contributing to new drug approvals. In January 2015, the FDA implemented its Drug Trials Snapshots program to make clinical trial demographic data more transparent and available.
Recently, the FDA took important steps to focus on inclusion in clinical trials with the release of their draft guidance for industry in April 2022 titled “Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials.”
“If you do not have a plan to drive enrollment for 40 percent of the US population who identify as racial or ethnic minorities, you are already starting with the glass half full. Enrollment will be slower, and efficiency will not be what was expected,” says Dr. Satya Shreenivas, MD, Medical Director from the global clinical research organization (CRO) Medpace.
In a recent webinar, Dr. Shreenivas described the trends in the diversity of the US population and why diversity and equality in clinical research needs to be a top priority. In the same webinar, Dr. Hansie M. Mathelier, MD, FACC, Clinical Assistant Professor of Medicine, Penn Medicine, spoke about patient diversity in cardiovascular clinical trials and the barriers to patient diversity in clinical research. The webinar concluded with Dr. Sara Sampaio, MD, CCRP, Manager, Patient Recruitment, Medpace, describing the solutions to promote diversity, equity and inclusion in clinical trials.
Watch the on-demand webinar to gain valuable insights from a team of experts about the challenges and considerations for recruiting and retaining a diverse patient population in clinical trials.
The Diversity of the US Population
The racial and ethnic makeup of the US is changing. For instance, the proportion of White (non-Hispanic) people in 2010 was 63.8 percent and in 2020 it was less than 60 percent (Figure 1). In 2020, the largest racial and ethnic groups after the White (non-Hispanic) population was Hispanic/Latino (19.6 percent), Black (12.6 percent) and Asian (5.9 percent; Figure 1).
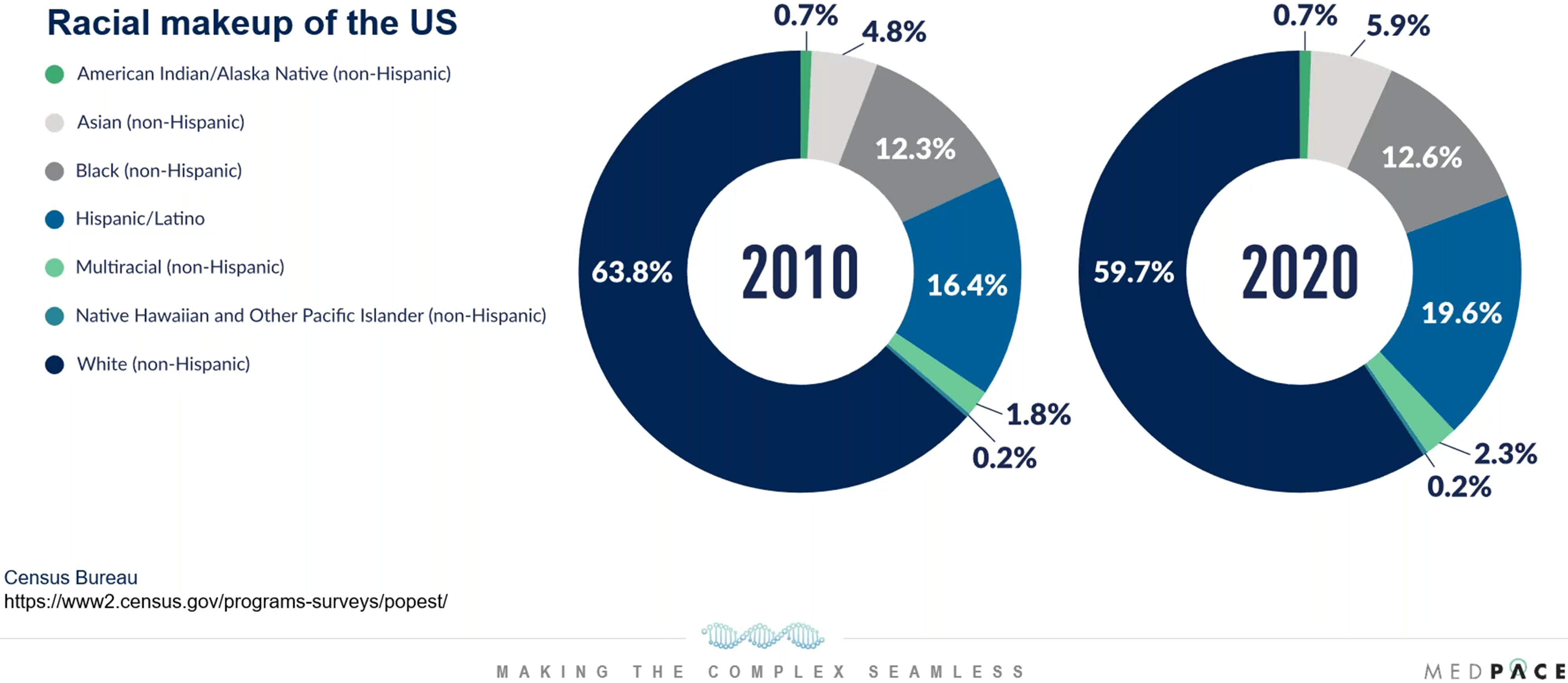
Figure 1. How has the racial and ethnic makeup of the US changed?
It is important to consider how the US racial and ethnic populations may change in the future because on average, an investigational product can take five to seven years to undergo Phase I to Phase III development, and this is ideally followed by at least 10 to 15 years of commercial value.
It is predicted that by 2045, more than 50 percent of the US population will be other than White (non-Hispanic). Therefore, the US population will change and become more diverse. As a result, there must be a plan to promote enrollment of diverse patient populations to ensure appropriate representation in clinical research and clinical trial efficiency.
Population diversity in the US varies considerably by geographic location and the expected increase in diversity will likely not be a uniform change across the country (Figure 2).
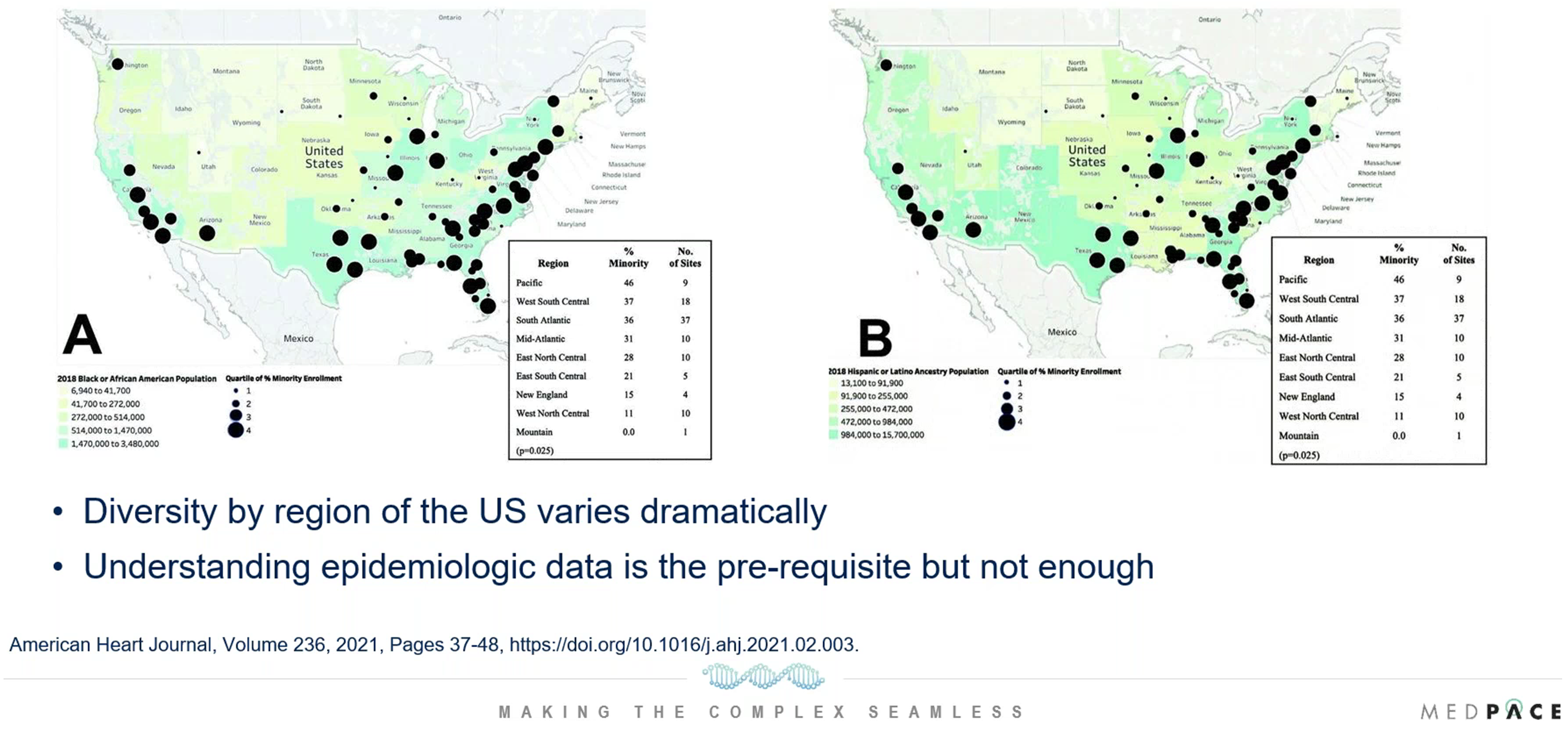
Figure 2. Diversity differences by geography. The density of the Black US population is on the left and the density of the Hispanic or Latino ancestry population is on the right.
During the webinar, Dr. Shreenivas mentioned an example of a cardiovascular clinical trial that took into consideration different geographic trends in population diversity. The National Institutes of Health (NIH)-funded Framingham Heart Study is a long-term clinical trial involving residents of Framingham, Massachusetts, who are mostly White. This prompted the NIH to focus on the Black US population to define risk factors and understand cardiovascular disease better, and as a result the Jackson Heart Study was set up in Jackson, Mississippi because that is where the target patient population is located.
“You go where the patients are if you want to ensure success and understanding that based on the different geographic trends is really important when you come up with your diversity plan,” says Dr. Shreenivas.
Age distribution is also a factor to consider when designing a clinical trial around patient diversity. For example, 25 percent of the US Black population is Generation Z and population diversity by age can vary (Figure 3).
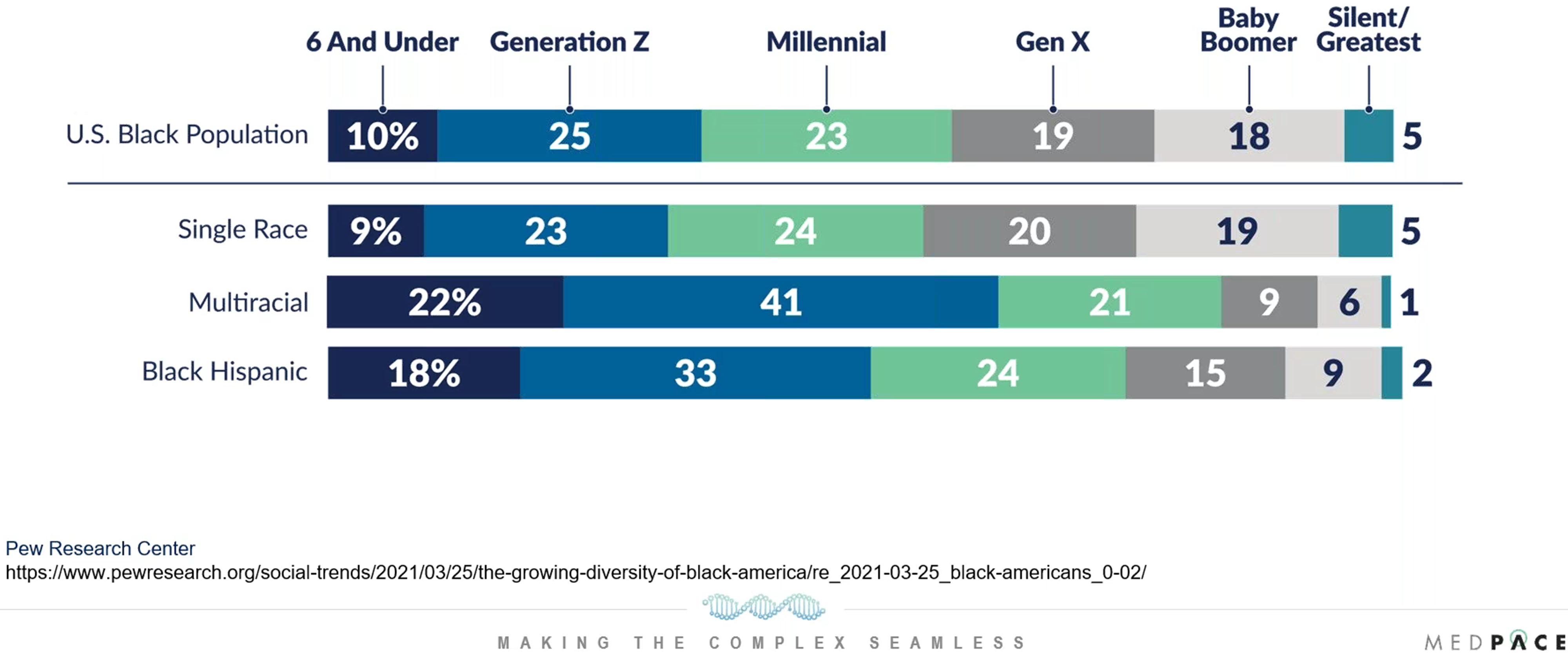
Figure 3. Diversity differences by age.
“If you want to do a hypertension or hyperlipidemia trial where the enrollment ranges 18 to 80, age distribution by race might not matter as much. But the vast majority of the Black and Hispanic population fit under the Millennial and Generation Z population. These ranges are incredibly important if you are aiming for a therapy for a rare disease in an older patient population. Therefore, taking race, geography and age into account is incredibly important,” explaines Dr. Shreenivas.
Why Should We Care About Diversity and Equity in Clinical Research?
To get the right drug or device to the right patient at the right time, clinical trials need to have a diversity plan because there can be a difference in disease burden when comparing people of different race and ethnicity.
During the webinar, Dr. Shreenivas gave examples from cardiology and oncology which show that race and ethnicity do matter for medical problems. For instance, Dr. Shreenivas showed data collected by the US Cancer Statistics Working Group from 1999 to 2018 which indicate that the US Black population is more pre-disposed to dying of cancer compared to White, Hispanic, Asian/Pacific Islander and American Indian & Alaska Native populations (Figure 4).
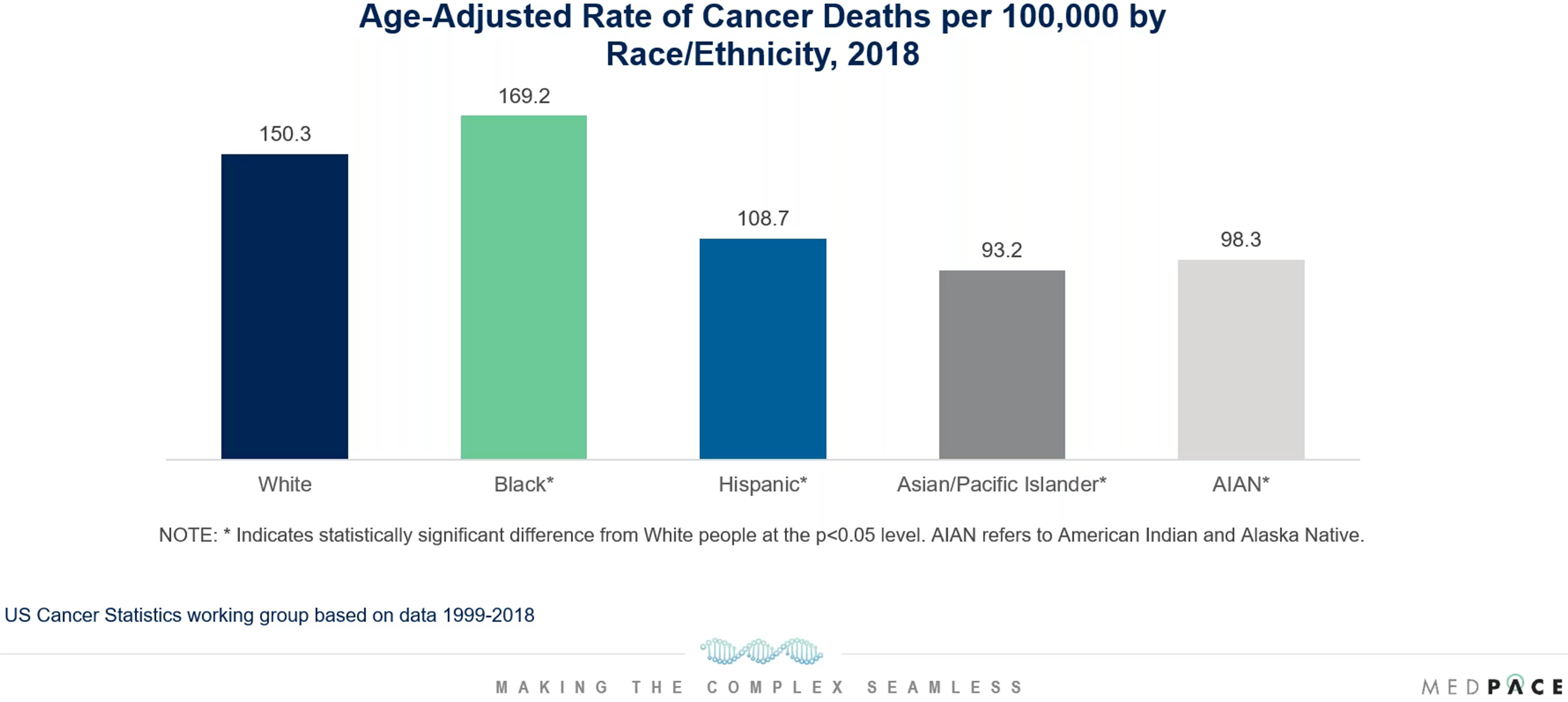
Figure 4. Does race matter for medical problems? An example from oncology.
Additionally, Dr. Shreenivas explained that the burden of cardiovascular disease can differ by race. In 2016, the cardiovascular disease mortality rate among non-Hispanic Black people was 211.6 per 100,000 in the US, which was higher than all other racial/ethnic groups. Despite their greater disease burden, Black, Indigenous and People of Color (BIPOC) remain underrepresented in cardiovascular outcome trials (Figure 5).

Figure 5. What is happening by race in clinical trials? Data reported in 2011 shows that Hispanics and African Americans comprised 16 percent and 12 percent of the US population, respectively, but only one percent and five percent of trial participants were Hispanics and African Americans, respectively.
Furthermore, the lack of diversity is more pronounced in industry-funded trials compared to NIH-funded trials (Figure 6). This is because in 2001 the NIH set a policy and guidelines for the inclusion of women and minorities in NIH-funded clinical research.
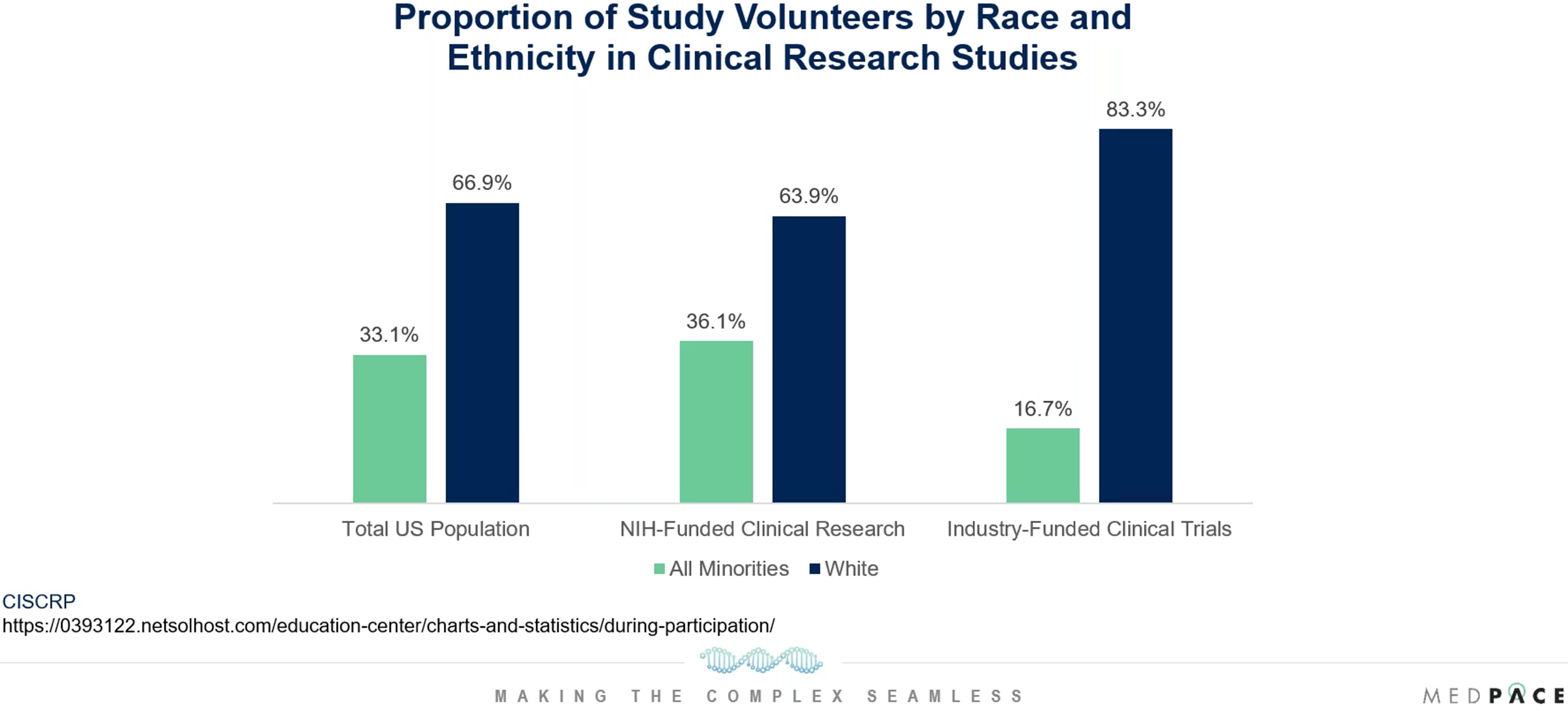
Figure 6. Lack of diversity is more pronounced in industry-funded trials.
This gives hope that the diversity of the US population can be mirrored in clinical trials if the life sciences industry focuses more on diversity and inclusion.
The Current Trends in Patient Diversity in Cardiovascular Clinical Trials
During the webinar, Dr. Mathelier discussed the results of several recent peer-reviewed publications that analyzed patient diversity in cardiovascular clinical trials.
In a study published in 2020 by the Journal of the American Heart Association, the authors evaluated the 10-year trends in enrollment of women and minorities in trials that supported FDA approvals of 35 new cardiometabolic drugs from 2008 to 2017. During the study period, the FDA approved 24 novel cardiovascular drugs and 11 novel diabetes mellitus drugs, and the median number of participants supporting each drug was 5930 individuals. Among the trial participants, 81 percent were White non-Hispanic, 12 percent Asian, 11 percent Hispanic/Latino and 4 percent Black (Figure 7). Unfortunately, the authors indicated that there was no significant improvement in the enrollment of racial or ethnic minorities over the analyzed period.
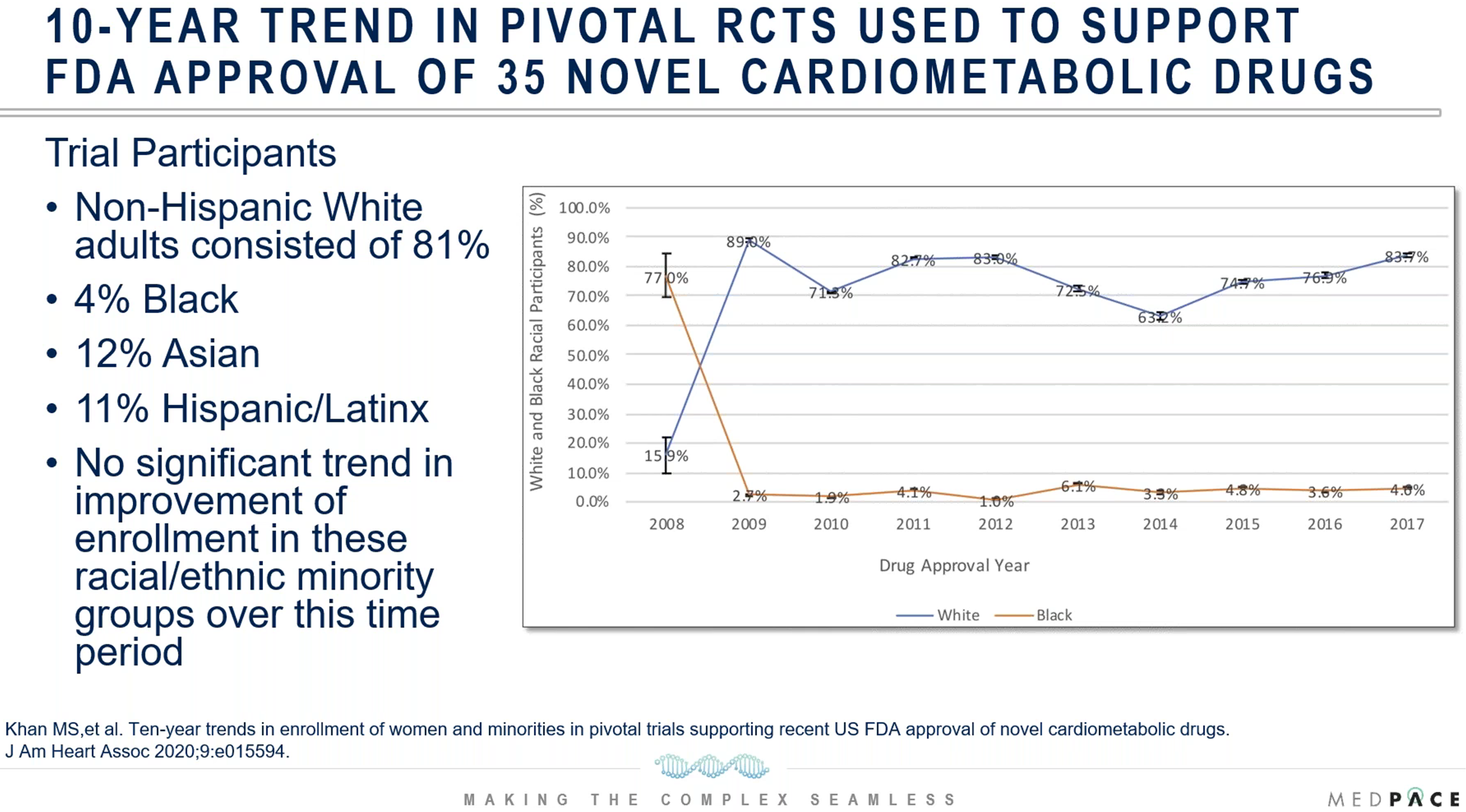
Figure 7. An analysis of trials that supported FDA approvals of new cardiometabolic drugs from 2008 to 2017.
A study published in 2021 in JAMA Network Open evaluated the representation of Black US individuals in pivotal clinical trials of 24 cardiovascular drugs which were given FDA approval from 2006 to 2020. The authors calculated the participation to prevalence ratio (PPR) and a PPR smaller than 0.8 or greater than 1.2 implies that certain racial or ethnic groups are underrepresented or overrepresented, respectively.
The results revealed that the percentage of clinical trial participation of Black US residents was only 2.9 percent and the total PPR for all cardiovascular conditions was only 0.29. The lowest PPR for Black participants was 0.072 for a hypercholesterolemia trial and the highest PPR was 0.52 for a hypertension trial. All PPRs for Black US residents were less than 0.8 while all PPRs for White participants were greater than 0.8 (Figure 8). Therefore, Black US residents were underrepresented in those clinical trials relative to their disease burden in the general population.

Figure 8. Black US residents were underrepresented in the analyzed cardiovascular clinical trials relative to their disease burden in the general population.
“More effective strategies to enhance the enrollment of Black participants in cardiovascular clinical trials can include a 10 percent to 50 percent accrual rate of minority groups in clinical trials. This is mostly based on oncology literature,” says Dr. Mathelier. “Multiple studies have shown that high enrollment of underrepresented populations did not influence study performance metrics.”
History and Observations of Pain Points that have been Barriers to Diversity in Clinical Trials
During the webinar, Dr. Mathelier used information in a systemic review article to discuss examples of distinct barriers that different racial and ethnic groups may have which hinder them from participating in trials:
- African Americans — legacy of mistrust
- Lack of research integrity
- Legacy of racism and discrimination
- Mistrust of healthcare system
- Concerns with research process
- Historical abuse that involved unethical research, lack of transparency and voluntary informed consent, such as the legacy of Tuskegee and the legacy of Henrietta Lacks
- Asian American
- Lack of family support on decision to participate
- Acculturation
- Pacific Islanders
- Misrepresentation of community
- Overgeneralization
- Hispanic/Latino
- Clinical trial content may not be culturally sensitive
- Differences in primary language
- Translators may distort the relationship between study team and patient
Dr. Mathelier explained that other barriers to participation among racial and ethnical groups may involve language and cultural differences, health literacy, religion, limited access within the healthcare system, lack of clinical trial awareness and uncertainty about what it means to participate in a trial.
Unfortunately, other barriers to patient diversity can involve clinical trial referral and recruitment biases. In 2010, interviews about minority enrollment were conducted in cancer centers affiliated with the Consortium for Enhancing Minority Participation in Clinical Trials. During the webinar, Dr. Mathelier reviewed the qualitative research findings that were gathered during these interviews of staff responsible for referring patients to potentially appropriate studies. The repetitive themes discussed by clinical trial administrators were:
- Interactions with potential minority participants were perceived to be challenging
- Potential minority participants are not perceived to be ideal study candidates
- A combination of clinic-based barriers and negative perceptions of minority study participants leads to clinicians withholding clinical trial opportunities from potential minority participants
- When clinical trial recruitment practices were tailored to minority patients, addressing misconceptions to build trust was a common strategy
- For some respondents, race was viewed as irrelevant when screening and recruiting potential minority participants for clinical trials
Therefore, negative stereotypes about the participation of minorities in clinical trials and the reluctance of staff to discuss the influence of race and ethnicity are hampering the commitment to improve recruitment of underrepresented participants.
Solutions to Promote Diversity, Equity and Inclusion: A Collaborative Effort Between the CRO Medpace, the Sponsor and Sites
The clinical research organization (CRO) Medpace suggests that a united effort between the CRO, the sponsor and sites is vital for enhancing patient diversity.
During the webinar, Dr. Sampaio discussed four focus areas for achieving diversity, equity and inclusion (DEI) in clinical research (Figure 9).
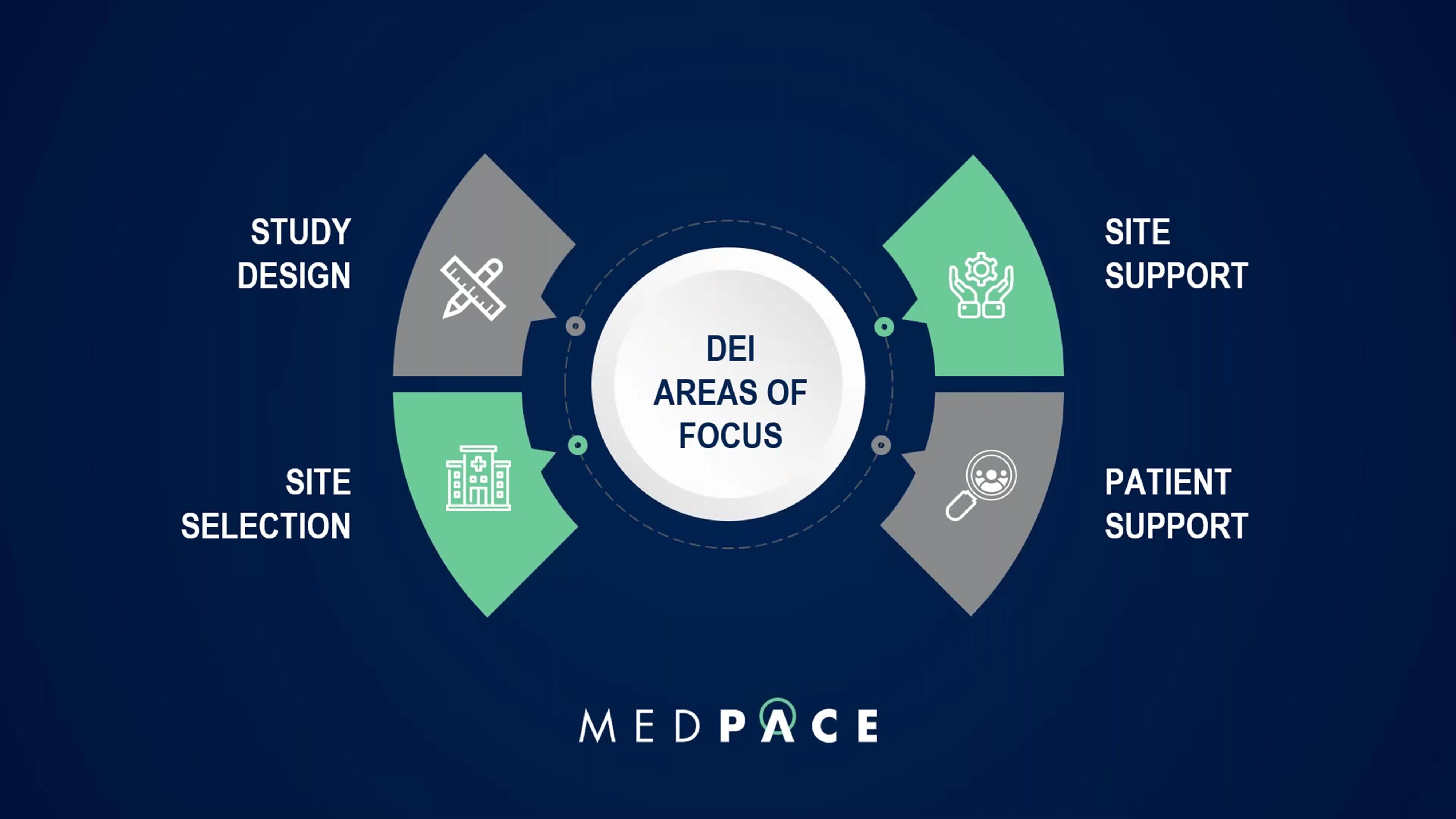
Figure 9. The CRO Medpace has four focus areas to achieve diversity, equity and inclusion (DEI) in clinical research.
Study Design Considerations
The earliest stages of planning the study design need to consider approaches for DEI. Trial methodologies should be flexible, and study procedures need to consider location, transportation, frequency of visits and potential financial barriers.
“There are methodologies that can be explored to allow more flexibility regarding eligibility criteria, for example, adaptive designs, broadening the inclusion criteria and evaluating exclusions that may preclude the enrollment of underrepresented groups,” says Dr. Sampaio. “For example, in heart failure trials a common inclusion criterion is the level of proBNP and we know there are racial variations in the level of proBNP.”
When permitted, study designs that improve accessibility are also beneficial for patient diversity. These include trials with technology alternatives (i.e., wearables that allow for remote patient sensing) or decentralized trials.
Considerations for Site Selection
Patient diversity based on geographic location is frequently considered when selecting sites for clinical research. However, less attention may be paid to the diversity of the research team at the site, even though it is beneficial if PIs, recruiters and other research staff can engage participants in multiple languages.
“Patients will need to feel represented, and we know that patients may be more inclined to enroll and follow up when they can interact better with the research team,” says Dr. Sampaio.
In addition, other site selection criteria to take into mind are the amount of referral sources from other areas and the level of community engagement with the site.
Site Support Considerations
Sites need continuous support during the trial to make sure patient recruitment and retention is going well. Medpace supports sites by providing services, education, resources and incentives to promote DEI. The examples that Dr. Sampaio mentioned during the webinar are:
- DEI awareness and training: tools and resources
- Study documents in multiple languages
- Translation services
- Patient concierge services
- Incentives and support for sites to engage in community health fairs to provide education about clinical research, trial indication and conduct pre-screening
Considerations for Patient Support
“The number one reason for declining participation in research is due to the inconveniences of travel,” states Dr. Sampaio.
For this reason, Medpace offers patient concierge services (travel and reimbursement) as well as home and telehealth options when allowed.
Making sure that patients feel heard and earning the trust of patients can greatly impact DEI in clinical research. One way that Medpace fosters patient support is by encouraging staff to include caregivers or close family members in discussions and study updates.
Dr. Sampaio explains that Medpace also invests in broad educational tools for patients while keeping in mind low health literacy, language and culturally sensitive approaches. Medpace also promotes patient engagement and seeks participant feedback to make sure patients are involved and supported.
A key message from the webinar was that patient diversity in clinical trials is achievable with a well-thought-out diversity plan and close collaboration between the CRO, sponsor and sites.
To learn more about the challenges and considerations for recruitment and retention of diverse patient populations, register to watch the free on-demand webinar by Medpace.
This article was created in collaboration with the sponsoring company and the Xtalks editorial team.





Join or login to leave a comment
JOIN LOGIN