Researchers at the University of California (UC), San Diego, have developed bone tissue which could one day be used to treat patients with various types of bone marrow disease. The engineers published their work in the journal, PNAS.
Currently, patients who are set to receive a bone marrow transplant must first undergo radiation to eliminate the patient’s own stem cells present in the bone marrow. Without this pretreatment, the donor cells would need to compete with the endogenous stem cells, significantly reducing their chances of survival.
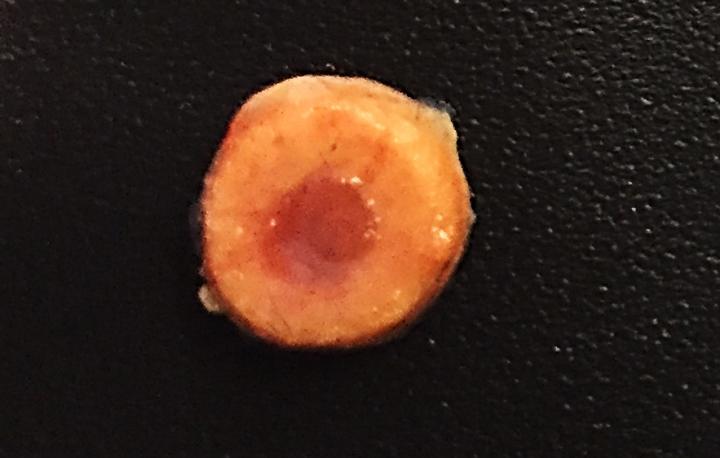
However, radiation treatment comes with a whole host of side effects, including fatigue, nausea and even loss of fertility. In an attempt to improve this transplant process, the researchers developed biomimetic bone tissue containing the donor cells, which could be implanted into a patient. This engineered tissue could act as an incubator for the transplanted cells, allowing them to grow and divide, and eliminated the need for radiation treatment to kill off the patient’s endogenous cells.
“We’ve made an accessory bone that can separately accommodate donor cells,” said senior author Dr. Shyni Varghese of the UC San Diego Jacobs School of Engineering. “This way, we can keep the host cells and bypass irradiation.”
The researchers tested the engineered bone tissue by filling it with donor cells and implanting it under the dermal surface of mice. Varghese and her team found that the implanted cells were able to survive in the mice for a minimum six-month period, slowing releasing new blood cells into the body.
“In the future, our work could contribute to improved therapies for bone marrow disease,” said primary study author Yu-Ru (Vernon) Shih, a research scientist in Varghese’s lab. According to Varghese, this implant would only be applicable to patients with non-cancerous forms of bone marrow disease, such as aplastic anemia. Patients with blood cancers – such as leukemia – would still need to undergo radiation treatment in order to eliminate the cancerous cells in the bone marrow.
The engineered bone implants are composed of a porous hydrogel matrix, designed to support the growth and differentiation of stem cells into blood cells. The outer mineralized matrix mimics that of long bones, allowing stem cells there to differentiate into bone-building cells.
“We’re working on making this a platform to generate more bone marrow stem cells,” said Varghese. “That would have useful applications for cell transplantations in the clinic.”










Join or login to leave a comment
JOIN LOGIN