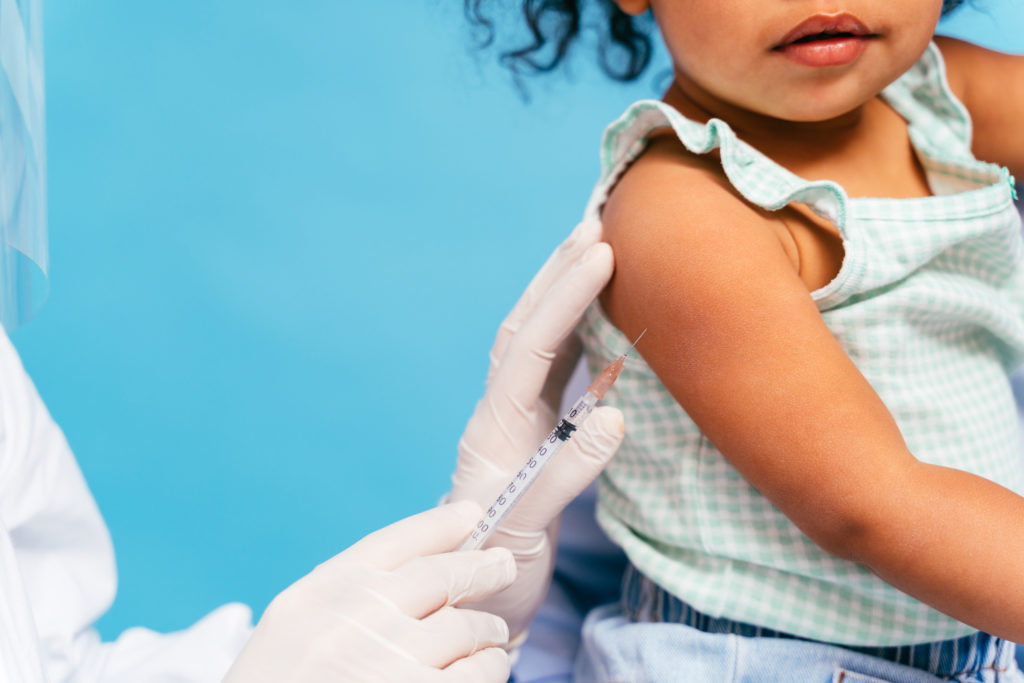
The US Food and Drug Administration (FDA) has authorized the use of Pfizer-BioNTech’s and Moderna’s COVID-19 vaccines for children as young as six months old, a much-awaited decision for some parents with young children.
The amended authorization came after FDA advisors on the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) unanimously backed the use of the vaccines in young age groups.
In that meeting, the committee members voted unanimously in favor of expanding the authorizations to include children as young as six months.
The FDA’s amendment to Moderna’s emergency use authorization (EUA) includes authorization of its vaccine for individuals six months through to 17 years of age. The vaccine had been authorized for use in adults 18 years of age and older.
Pfizer-BioNTech’s revised authorization for its COVID-19 vaccine now includes use in individuals six months through four years of age. The vaccine had been authorized for use in individuals five years of age and older.
In a press announcement outlining the amended authorizations, the FDA said its “evaluation and analysis of the safety, effectiveness and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs.”
Following the FDA authorization, the Centers for Disease Control and Prevention (CDC) provided its recommendation and guidance for the COVID-19 vaccines for everyone six months and older, and boosters for everyone five years and older (if eligible).
Related: Novavax COVID-19 Vaccine Gets Recommended for FDA Emergency Use Authorization
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children and this action will help protect those down to 6 months of age. As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death,” said FDA Commissioner Robert M. Califf, MD.
“Those trusted with the care of children can have confidence in the safety and effectiveness of these COVID-19 vaccines and can be assured that the agency was thorough in its evaluation of the data,” explained Dr. Califf.
The Pfizer-BioNTech vaccine is administered in individuals six months through four years of age as a primary series of three doses, with the first two doses given three weeks apart followed by a third dose administered at least eight weeks after the second dose.
The primary series of Moderna’s vaccine in individuals six months to 17 years of age consists of two doses administered one month apart. A third primary dose, given at least one month following a second dose, is authorized for individuals in this age group who have certain types of immunocompromise.
The FDA’s decision on the amended vaccine authorizations for the pediatric groups are based on effectiveness and safety data that the agency analyzed and evaluated.
Moderna’s data came from two ongoing, randomized, blinded, placebo-controlled trials being conducted in the US and Canada, which includes infants, children and adolescents. In a subset of 230 children six months through 23 months of age, the immune response after receiving a two-dose primary series of 25 micrograms of the Moderna mRNA vaccine was comparable to the immune response of adults (18 to 25 years of age who received two higher doses of the vaccine in a previous study).
Similarly, analyzed trial data for the Pfizer-BioNTech mRNA vaccine showed that in a subset of children aged six months to four years who received three doses of the vaccine achieved an immune response comparable to that in older individuals (adults 16 to 25 years of age).
Immune responses after receiving the Pfizer or Moderna vaccines in children six to 11 years of age were also comparable to older individuals.
Safety
With respect to safety of Moderna’s COVID-19 vaccine, among about 1,700 children six months through to 23 months of age and 3,000 children two years to five years of age who received the vaccine, the most commonly reported side effects were pain, redness, swelling at injection site, fever and swelling/tenderness of lymph nodes in the arm or thigh where the injection was administered. In the six months to 23 months age cohort, 1,100 of the participants were followed for at least two months for safety after receipt of the second dose.
Pfizer’s safety data included approximately 1,170 participants six months to 23 months of age who received the vaccine of whom 400 were followed for safety for at least two months following the third dose. Approximately 1,800 participants two to four years of age received the vaccine and 600 were followed for safety for at least two months following the third dose. The most common side effects reported among participants in both pediatric age groups who received the vaccine were irritability, decreased appetite, fever and pain, tenderness, redness and swelling at the injection site. Some additional reported side effects in the two to four years of age group included fever, headache and chills.
The low risks of cardiac inflammation, myocarditis and pericarditis associated with the mRNA COVID-19 vaccines, which are highest in older male adolescents and particularly after the second dose of Moderna’s vaccine, continue to be monitored by the FDA’s and CDC’s safety surveillance systems.
The FDA said, “the agencies to strengthen the evidence that most cases of myocarditis associated with the Moderna and Pfizer-BioNTech COVID-19 vaccines are characterized by rapid resolution of symptoms following conservative management, with no impact on quality of life reported by most patients who were contacted for follow-up at 90 days or more after reporting myocarditis.”
Vaccine Uptake
The authorization of the vaccines in younger populations is being met with mixed responses. While many parents are breathing sighs of relief, others remain hesitant. According to polling data from the Kaiser Family Foundation (KFF) from May, only one in five parents were willing to vaccinate children under the age of five.
About 22 percent of parents said the delay in the authorization for kids under five has made them “more confident” in the vaccine’s safety for young children, while around 13 percent said it has made them “less confident.”
The FDA says it has “determined that the known and potential benefits of the Moderna and Pfizer-BioNTech COVID-19 vaccines outweigh the known and potential risks in the pediatric populations authorized for use for each vaccine.”











Join or login to leave a comment
JOIN LOGIN