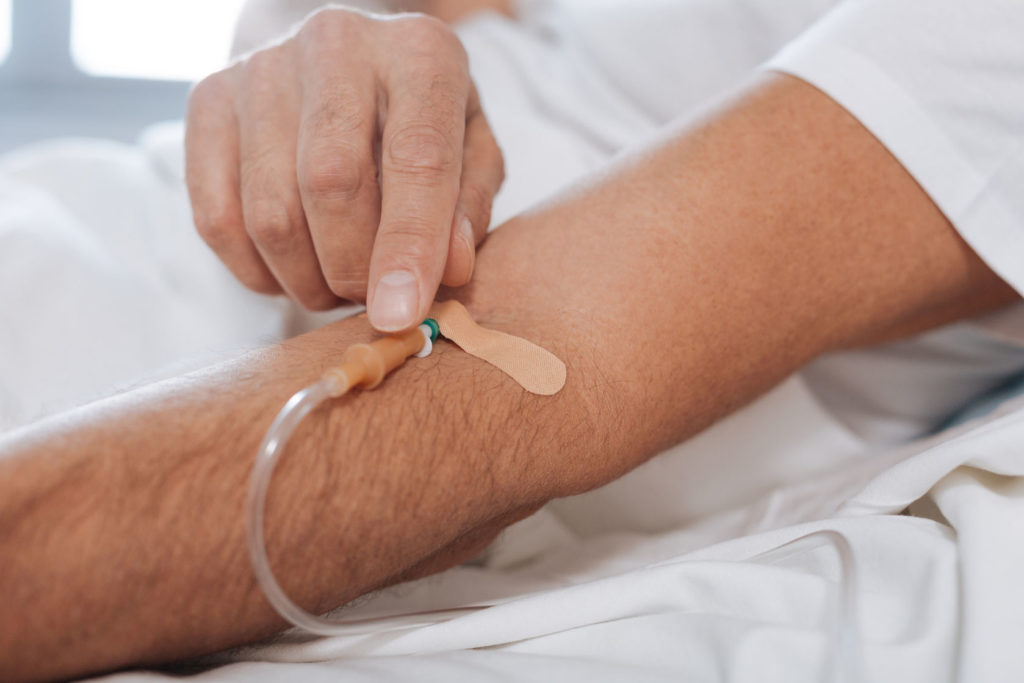
For the first time, US scientists are attempting to use gene editing to cure a man with a rare disease known as Hunter syndrome. Brian Madeux, a 44-year-old California man is the first person in the US to receive the experimental treatment which involves injecting billions of copies of a corrective gene along with zinc finger nucleases, which are enzymes designed to replace the faulty gene in his genome with the correct one.
When asked about how it feels to be the first to test this new technology, Madeux answered: “It’s kind of humbling. I’m willing to take that risk. Hopefully it will help me and other people.”
Less than 10,000 individuals around the world suffer from metabolic diseases, like Hunter syndrome, which primarily affects males. Patients with this condition lack the enzyme iduronate-2-sulfatase (I2S), which prevents them from being able to properly metabolize specific carbohydrates.
The build-up of glycosaminoglycans (GAG) in the cells leads to a number of symptoms, such as frequent colds, hearing loss, heart problems and even brain damage. While enzyme replacement therapy is somewhat effective, it can cost upwards of $400,000 per year for weekly treatment.
Gene therapy is an emerging field with a lot of potential to treat – and even cure – genetic diseases. But the much-hyped gene editing techniques, such as CRISPR, have largely only been tested on cell cultures in the lab.
The therapy used to treat Madeux includes the new, correct version of the gene that’s mutated in Hunter syndrome as well as two zinc finger nucleases, which are delivered into cells via a non-pathogenic viral vector. Once administered via an IV, the gene therapy is targeted towards the liver where cells synthesize the zinc finger proteins.
Those proteins then cut the DNA at a specific spot and replace the excised fragment with the corrective version of the gene. Once the edit is complete, the cells are able to synthesize the protein missing in patients with Hunter syndrome.
According to Madeux’s physicians, only one percent of the cells in his liver need to be edited in order to treat the disease. Madeux will have to wait three months to see if the gene therapy was successful.
However, there are potential risks to this type of treatment. The viral vector used to get the gene into the system could provoke a strong immune response, or the gene editing could have unintentional “off-target” effects whereby the replacement gene is inserted into other areas in the genome.
“When you stick a chunk of DNA in randomly, sometimes it works well, sometimes it does nothing and sometimes it causes harm,” said Hank Greely, a Stanford University bioethicist. “The advantage with gene editing is you can put the gene in where you want it.











Join or login to leave a comment
JOIN LOGIN